Abstract
Black shank, caused by Phytophthora nicotianae (P. nicotianae), is a serious disease of cultivated tobacco (Nicotiana tabacum) worldwide. The interactions between tobacco and P. nicotianae are complex and the outcomes of the interactions depend on the tobacco genotype, P. nicotianae strain, and environmental conditions. In this study, we used RNA-sequencing (RNA-Seq) to investigate and compare transcriptional changes in the stems of tobacco upon inoculation with P. nicotianae strain race 0. We used two tobacco varieties: RBST (named from resistance to black shank and tobacco mosaic virus), which was resistant to the P. nicotianae strain race 0, and Honghuadajinyuan (HD), which was susceptible to P. nicotianae race 0. Samples were collected 12 and 72-hour post inoculation (hpi). Analysis of differentially expressed genes (DEGs) and significantly enriched GO terms indicated that several basic defense mechanisms were suppressed in both varieties, which included response to wounding (GO: 0009611), and defense response to fungus (GO: 0050832). We also found some genes that may especially be related to mechanisms of resistance in RBST, such as the one encoding a chitinase. These results will provide a valuable resource for understanding the interactions between P. nicotianae and tobacco plants.
Similar content being viewed by others
Introduction
Black shank, caused by Phytophthora nicotianae (P. nicotianae), is among the most widespread and damaging diseases of cultivated tobacco (Nicotiana tabacum) worldwide1. The pathogen infects roots, stems, and leaves at any stage of plant growth, resulting in root rot, stem lesions, leaf necrosis, and plant death. Disease damage is increased rapidly under conditions of high temperature2, 3.
Management of tobacco black shank has relied upon an integrated approach that includes crop rotation, fungicide applications, and the use of resistant cultivars. The most cost-efficient method of managing the disease is the use of resistant cultivars4. Described physiological races of P. nicotianae include races 0, 1, 2, and 3, with races 0 and 1 currently having the highest prevalence5. Previous studies using tobacco cultivars with moderate or high levels of resistance have found that race 0 has higher pathogenic and ecologic fitness levels than race 1, suggesting that more attention should be paid to race 06. Complete resistance to race 0, the most common race of P. nicotianae, is found in Nicotiana plumbaginifolia 7. N. plumbaginifolia is a wild tobacco species that is generally used as a parent in genetic breeding programs. Such cultivars with this type of resistance are also immune to race 0.
The knowledge of resistant mechanisms of tobacco to P. nicotianae is limited. The mechanism of resistance is complex and may be related to an array of physiological processes, such as affecting infection effectiveness, influencing recognition between pathogens and their hosts, blocking hyphal expansion, and extracellular accumulation of a cytotoxic activity8,9,10. Understanding the response of tobacco to P. nicotianae is important for developing strategies for disease control. As complicated as the mechanisms of disease resistance in plants can be, it is generally accepted that global investigation of gene expression profiles during disease infections could help to identify key components of resistance pathways11. So far, there has not been any report on the transcriptome of tobacco in response to P. nicotianae infection. Two tobacco varieties, Honghuadajinyuan (HD) and RBST, show different resistant levels to P. nicotianae. Here we performed RNA sequencing analysis of gene expression profiles in both HD and RBST tobacco stems, which were infected with P. nicotianae at two time points: 12 h and 72 h post inoculation. By comparing the gene expression patterns of HD and RBST, we found that some plant defense genes were differentially expressed between the two varieties. The acquired transcriptome data provide an invaluable resource for understanding the response/resistance to P. nicotianae infection in tobacco.
Results
Symptoms of black shank on tobacco
Symptoms were not apparent on HD and RBST at 12 h after inoculation. At 72 h, the inoculation site on the HD stem turned from brown to black; the darkening extended up and down for several centimeters, and the plant was wilted. The inoculation site on the RBST stem also turned from brown to black at 72 h, but the darkening did not spread, and the plant grew well.
Annotation of unigenes in tobacco
We first defined the transcriptome by RNA-Seq. A total of 946,093,872 clean reads (141.92 Gb) were generated by Illumina RNA-Seq deep sequencing (Table 1). The reads of all samples (inoculated RBST, non-inoculated RBST, inoculated HD, and noninoculated HD) were pooled to generate a de novo assembly of transcriptomes. Initial assembly using Trinity yielded 316,948 contigs with an N50 value of 732 bp. Further prediction of open reading frames using TransDecoder obtained 49,935 unigenes, with an N50 value of 2,112 bp and a GC content of 40.05%. The N50 value was similar to those reported in other de novo assembly studies of tobacco12. We performed gene function annotation analysis of the 49,935 unigenes. The transcripts were aligned to the NCBI Non-Redundant Protein Database (NR database) using the blastp program (E-value 1e−5), allowing the annotation of 45,234 unigenes based on 32,161 NCBI non-redundant proteins. There were 39,127 unigenes that were represented by nearly full-length NR proteins, having > 80% alignment coverage. The Gene Ontology database was then utilized to retrieve the GO identifiers for each of the annotated genes. In total, 34,065 unigenes mapped to GO terms. We identified 49 main GO terms in three levels (23 GO terms in Biological Process, 14 GO terms in Molecular Function and 12 GO terms in Cellular Component) (Fig. 1).
The amount of P. nicotianae in the infected samples
It is unknown whether there were different trends for the change in the number of the pathogen during its infection of different tobaccos, we analyzed the read numbers that were derived from P. nicotianae. There were 1.9% and 2.3% reads were derived from P. nicotianae for HD in 12 h and 72 h. There were 1.4% and 2.2% reads were derived from P. nicotianae for RBST in 12 h and 72 h. The late stage of the infection had more reads of P. nicotianae than that at the early stage. The trends were similar for the two tobaccos. However, the susceptible variety HD had more reads of P. nicotianae than that the resistant variety RBST had at the early stage of infection.
Differentially expressed genes for each variety at two time points after infection
The gene expression profiles of healthy HD and RBST stems were used as controls. All genes and their gene expression data are listed in Supplementary Table S1 and Supplementary Table S2. If the gene expression in infected stems recorded a 4-fold (or more) difference relative to the control (P < 0.05), this gene was regarded as the differentially expressed gene (DEG).
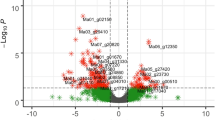
For HD, there were 2,778 DEGs at 12 h, and 2,728 DEGs at 72 h after inoculation. The number of upregulated DEGs at 12 h after inoculation was 1,368, and 1,152 at 72 h (Fig. 2a). For RBST, the number of DEGs decreased from 691 at 12 h after inoculation to 550 at 72 h after inoculation. The number of upregulated DEGs was lower than that of downregulated DEGs at 12 h (121 vs. 570 genes) and 72 h (58 vs. 492 genes) after inoculation (Fig. 2b).
As shown in Fig. 2, a Venn diagram was generated from the DEG lists at 12 h, and 72 h after inoculation to identify shared members. There were 2,011 differentially expressed genes at all time points in HD. The number of upregulated and downregulated DEGs were 842 and 1,169, respectively. For RBST, there were 373 DEGs that could be identified at all-time points. The number of upregulated and downregulated DEGs were 20 and 353, respectively.
As shown in Fig. 2, for HD, there were 767 DEGs that were only detected at 12 h and 717 DEGs that were only detected at 72 h. The numbers of upregulated DEGs were 526 at 12 h and 310 at 72 h, respectively. The numbers of downregulated DEGs were 241 at 12 h and 407 at 72 h, respectively. For RBST, there were 318 DEGs that only discovered in 12 h and 177 DEGs that were only discovered in 72 h. The numbers of upregulated DEGs were 101 at 12 h and 38 at 72 h, respectively. The numbers of downregulated DEGs were 217 at 12 h and 139 at 72 h, respectively.
Shared differentially expressed genes during P. nicotianae infection
The above-mentioned two lists of shared DEGs were further analyzed for commonalities and differences. As shown in the Venn diagram in Fig. 3, there were 264 common DEGs in two lists of DEGs. We were especially interested in the features of the 15-upregulated common DEGs (Supplementary Table S3). One gene, TR75953_c0_g12, was predicted to encode dehydration-responsive element-binding protein. This gene was involved in abscisic acid (ABA) -independent defense against pathogens in Arabidopsis thaliana and tobacco13, 14. Another gene, TR30085_c0_g2, encode a zinc finger protein. It participates in regulation of transcription. It is possible that it is important for the response to pathogens15. The functions of other DEGs were not clear.
Additionally, different numbers of unique DEGs were found for each variety: 1,747 unique DEGs in HD, and 109 unique DEGs in RBST. Especially for the resistant variety RBST, there were five upregulated DEGs (Supplementary Table S4). One gene, TR75736_c2_g1, which encodes a chitinase, is usually involved in the response to fungal pathogens16.
Gene ontology (GO) enrichment analysis of differentially expressed genes
Within 49,935 identified unigenes, the protein products of 34,065 unigenes were annotated with at least one GO term. Using these 34,065 unigenes as references, 526 unique upregulated DEGs in HD_12 h, and 310 unique upregulated DEGs in HD_72 h, were enriched in 53 and 41 GO terms, respectively. These 53 GO terms were involved in “plastid part (GO: 0044435)” and “chloroplast thylakoid (GO: 0009534)” (Supplementary Table S5). These 41 GO terms were involved in “biological regulation (GO: 0065007)” and “gibberellin biosynthetic process (GO: 0009686)” (Supplementary Table S6). Similarly, 101 unique upregulated DEGs in RBST_12 h, and 38 unique upregulated DEGs in RBST_72 h, were enriched in 6 and 6 GO terms (Supplementary Table S7). These GO terms at 12 h were involved in “cell junction assembly (GO: 0034329) and “secondary cell wall (GO: 0009531)”. The GO terms at 72 h were involved in “defense response, incompatible interaction (GO: 0009814)” and “innate immune response (GO: 0045087)”.
There were 264 common DEGs between HD and RBST including 15 upregulated and 249 downregulated DEGs. For upregulated DEGs, they were enriched in seven GO terms, such as “organelle (GO: 0043226)” (Supplementary Table S8). For downregulated DEGs, 119 GO terms were identified such as “response to wounding (GO: 0009611)” and “defense response to fungus (GO: 0050832)” (Supplementary Table S9).
Only five upregulated DEGs were specifically identified and two GO terms were specifically enriched in RBST. They were “exochitinase activity (GO: 0035885)” and “endochitinase activity (GO: 0008843)”.
Quantitative RT-PCR (qRT-PCR) validation of differentially expressed genes
A subset of 10 genes, which responded to P. nicotianae infection, were selected for quantitative real-time PCR (qRT-PCR) analyses (Supplementary Fig. S1). qRT-PCR analyses showed the trends of expression to be consistent with those found by RNA-Seq.
Discussion
Black shank, as one of the most important plant diseases, causes crop losses worldwide17. In order to identify tobacco genes involved in broad-spectrum resistance to tobacco black shank, a previous study using suppression subtractive hybridization (SSH) was performed in Nicotiana megalosiphon to identify differentially expressed cDNAs, and 48 differentially expressed genes were discovered18. In this study, we used next-generation sequencing approaches to investigate the gene expression changes associated with the characteristic disease development process induced in tobacco plants by P. nicotianae. The results will assist in the discovery and annotation of important genes in plant defense response, physiology and metabolism. RNA-Seq analysis identified a total of 49,935 unigenes in tobacco. GO analysis showed that 34,065 of the unigenes could be grouped into 49 known GO terms. Our analyses of GO terms generally represented the main biological GO classification and ensured the integrity of the downstream functional analyses of the candidate genes.
It is worth mentioning that various unique GO terms were enriched at different time points. For example, the cellular component GO terms plastid part (GO: 0044435) and chloroplast thylakoid (GO: 0009534) were only enriched in HD after 12 h of infection. At 72 h, GO terms biological regulation (GO: 0065007) and gibberellin biosynthetic process (GO: 0009686) were enriched. Close examination of these GO terms may provide molecular insight into the mechanism of response to pathogens at different times after infection. The chloroplast is a photosynthesis-related organelle. Photosynthesis has been reported to modulate plant defense responses induced by pathogen infection19. At 72 h, some new biological processes were enhanced, such as gibberellin biosynthetic process. Previous studies have proven that the function of plant hormone signal transduction plays an important role in the defense response20. It is interesting that increasing the content of gibberellin in N. benthamiana plants could enhance the susceptibility of these plants to infectious pathogens21. One gene, TR75953_c0_g12, was upregulated in the HD both at 12 h and 72 h. This gene was involved in abscisic acid (ABA)-independent defense against pathogens in Arabidopsis thaliana and tobacco13, 14. ABA has been considered a negative regulator of disease resistance. These may be some reasons why HD is susceptible to P. nicotiana. Similarly, some special GO terms for RBST were identified at different time points. Cell junction assembly (GO: 0034329) was enriched at 12 h, and innate immune response (GO: 0045087) was enriched at 72 h. Our results further revealed that several genes related to innate immunity, e.g., ADR1 and RPM1 22, 23, were also induced during systemic symptom development. These results indicated that the two varieties had different mechanisms to respond to P. nicotianae infection at different time points.
Our differentially expressed gene (DEG) analysis provided further insight into the molecular mechanism for common biological processes between resistant and susceptible tobaccos after P. nicotianae infection. Analysis of significantly enriched GO terms indicated that several biological processes are common between the two varieties. Most of the upregulated DEGs are enriched in cellular component GO terms, which were related to organelle construction. On the other hand, the downregulated DEGs belong to biological processes: response to wounding (GO: 0009611) etc. These downregulated biological processes belonged to defense-related pathways. And these pathways played important roles in defense response24. Those down-regulated genes in these pathways were likely to decrease basal defense response and reduce disease resistance to P. nicotianae infection in both resistant and susceptible tobaccos. It is well known that plant pathogens have evolved to secrete effectors, which can manipulate the host immune system and suppress host defense25. Previous papers have evidenced that some resistance (R) genes can be activated by specific effectors, which is shown by the accumulation of higher levels of R genes transcripts. Therefore, we paid attention to those putative R genes that could possibly be induced by P. nicotianae infection of the two varieties. Among these genes, TR29096_c1_g6 and TR29096_c0_g1 were commonly up-regulated in HD and RBST. These R proteins directly or indirectly detect bacterial or fungal effectors, which will activate downstream signaling and lead to pathogen resistance26.
The variety RBST has high levels of resistance to P. nicotianae race 0. However, why it could avoid the development of symptoms was not clear. In this study, we found some genes and biological processes that may be related to this resistant mechanism. Gene TR75736_c2_g1, which encodes chitinase, participated in exochitinase activity (GO: 0035885) and endochitinase activity (GO: 0008843). The expression level of this gene was 10 times higher than that of the control. In vitro assays in rice indicated that the chitinase showed antifungal activity with a clear inhibitory effect on the growth of the pathogen Rhizoctonia solani 27. The gene (TR24473_c0_g2) encoding plasma membrane protein, was upregulated in RBST. The plasma membrane is involved in important cellular process that determines signal response of plant to pathogen such as hypersensitive response28. There was no overlap between the 48 genes identified by SSH study18 and genes identified by our RNA-seq study. This may due to the different species used in these studies. The SSH study was performed in Nicotiana megalosiphon.
Genetic resources for resistance to black shank in the varieties of tobacco currently in use for production are mainly derived from Florida 30129 and N. plumbaginifolia 30. The varieties currently used to produce commercial flue-cured tobacco are not completely immune to black shank, and the effects can still be seen when pathogen levels exceed a critical threshold. Therefore, to reduce losses in tobacco production, it will be essential to perform more studies to identify genes and pathways involved in resistance to black shank.
In conclusion, the gene expression patterns of HD and RBST after P. nicotianae infection provide a solid foundation for future studies of the molecular mechanisms underlying the response of tobacco to black shank.
Materials and Methods
Inoculation of tobacco with P. nicotianae
The resistant tobacco breeding line RBST and the susceptible tobacco cultivar Honghuadajinyuan (HD) were used in the experiments. The two varieties of N. tabacum were developed by our institution. The detailed development processes of the two varieties had been described in the Supplement Materials and Methods. A field-isolate of P. nicotianae race 0 was used for all inoculations throughout this study. The inoculum and the protocol for inoculation under greenhouse conditions were prepared as described31. The infected stem was harvested at two time points, 12 and 72 h post inoculation.
Sample collection and library preparation
All three biological replicate samples were used for RNA extraction. Total RNA was extracted from inoculated as well as noninoculated plants using TRIzol reagent (Invitrogen Corp., Carlsbad, CA). RNA purifications were performed using an RNeasy Mini Kit (Qiagen, Chatsworth, CA). Library preparation was carried according to the Illumina Hiseq RNA sample preparation kit (Illumina, San Diego, CA). All original data were deposited in the NCBI Sequence Read Archive database (accession number: SRP074868).
De novo assembly and annotation of transcriptomes
Raw data (raw reads) in fastq format were first processed through the NGS QC Toolkit32. In this step, clean data (clean reads) were obtained by removing reads containing adapter, reads containing ploy-N, and low quality reads from raw data. At the same time, Q20, Q30, and GC-content of the clean data were calculated. All downstream analyses were based on clean data. The black shank resistance gene in RBST was derived from N. plumbaginifolia 30. The three varieties that had reference genomes do not have the introgression segment with resistance gene conferring resistance to P. nicotianae race 033. So de novo assembled transcriptome was necessary. The transcriptome was de novo assembled using Trinity with the default parameters34. The candidates with the most probable longest ORF were generated from the Trinity assembly result using TransDecoder (https://transdecoder.github.io/). Protein sequences corresponding to the coding sequences of unigenes were obtained and searched against the NCBI non-redundant (nr) database, using blastp with a cut-off E-value of 10−5. Gene Ontology (GO) annotation was based on the Gene Ontology Database of Arabidopsis thaliana35. WEGO software was used to perform functional annotation analyses at three gene ontology levels (Biological Process, Molecular Function and Cellular Component)36.
Detection of differentially expressed genes
RSEM37, an accurate transcript quantification tool for RNA-Seq data, was utilized to quantify transcripts. Briefly, clean data were mapped back onto the assembled transcriptome. The read count for each gene was obtained from the mapping results. Differential expression analysis between inoculated and noninoculated plants was performed using the DESeq38 and EdgeR39 package. The resulting P values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate (FDR)40. The overlap between the sets of genes found by DESeq and edgeR with FDR < 0.05 and the absolute value of Log2 (Ratio) ≥ 2 were assigned as differentially expressed genes (DEGs). Venn Diagrams were drawn with VENNY (http://bioinfogp.cnb.csic.es/tools/venny).
Gene ontology enrichment analyses
We identified GO terms at three levels (biological process, molecular function and cellular component). GO terms enriched in genes from the differentiation analyses were identified with KOBAS software41. GO terms with a false discovery rate (FDR)42 of less than 0.05 were considered over-represented.
Quantitative real-time PCR (qRT-PCR) validation
To validate the DEG results, 10 DEGs were randomly selected and qRT-PCR analysis was performed. Total RNA was isolated using a TRIzol kit (Invitrogen, USA). First-strand cDNA synthesis was performed using the PrimeScriptTM First Strand cDNA Synthesis Kit (Takara, Japan). The actin gene (GenBank no. X63603) was used as the internal control. Primer sets were designed using Primer Premier 6.0 software43 (Supplementary Table S10). qRT-PCR was performed usinga SYBR Green qPCR kit (New England Biolab) according to the manufacturer’s instructions. All qRT-PCR experiments were performed in triplicate using independent samples. Expression quantification and data analysis were performed using the 2−ΔΔCt method44.
Data availability
The RNA-Seq raw data were deposited in the NCBI Sequence Read Archive (SRA) with the accession number SRP074868.
References
Davis, D. L. & Nielsen, M. T. Tobacco: Production, Chemistry and Technology. (Blackwell Publ, 1999).
Lamour, K. Phytophthora: a global perspective. (CABI, 2013).
Csinos, A. S. Stem and root resistance to tobacco black shank. Plant Disease 83, 777–780 (1999).
Hu, J. H., Hong, C. X., Stromberg, E. L. & Moorman, G. W. Mefenoxam sensitivity and fitness analysis of Phytophthora nicotianae isolates from nurseries in Virginia, USA. Plant Pathology 57, 728–736 (2008).
Drake, K. E. et al. Black shank resistance and agronomic performance of flue-cured tobacco lines and hybrids carrying the introgressed nicotiana rustica region, Wz. Crop Science 55 (2015).
Sullivan, J. M., Melton, T. A. & Shew, H. D. Fitness of races 0 and 1 of Phytophthora parasitica var. nicotianae. Plant Dis 89, 1220–1228 (2005).
Chaplin, J. F. Transfer of black shank resistance from Nicotiana plumbaginifolia to flue-cured N. tabacum. Tob. Sci 6, 184–189 (1962).
Meng, Y., Zhang, Q., Ding, W. & Shan, W. Phytophthora parasitica: a model oomycete plant pathogen. Mycology 5, 43–51 (2014).
Hugot, K. et al. Coordinated regulation of genes for secretion in tobacco at late developmental stages: association with resistance against oomycetes. Plant physiology 134, 858–870 (2004).
Tyler, B. M. Molecular basis of recognition between phytophthora pathogens and their hosts. Annual review of phytopathology 40, 137–167 (2002).
Feys, B. J. & Parker, J. E. Interplay of signaling pathways in plant disease resistance. Trends in genetics: TIG 16, 449–455 (2000).
Duan, S. et al. Transcriptomic profile of tobacco in response to Alternaria longipes and Alternaria alternata infections. Sci Rep 6, 25635 (2016).
Fan, J., Hill, L., Crooks, C., Doerner, P. & Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant physiology 150, 1750–1761 (2009).
Mauch-Mani, B. & Mauch, F. The role of abscisic acid in plant-pathogen interactions. Current opinion in plant biology 8, 409–414 (2005).
Guo, Y. et al. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol 183, 62–75 (2009).
Hawkins, L. K. et al. Characterization of the maize chitinase genes and their effect on aspergillus flavus and aflatoxin accumulation resistance. PloS one 10, e0126185 (2015).
Li, B.-C., Bass, W. T. & Cornelius, P. L. Resistance to tobacco black shank in Nicotiana species. Crop Science 46, 554–560 (2006).
Chacón, O. et al. Identification of defense-related genes in tobacco responding to black shank disease. Plant Science 177, 175–180 (2009).
Hua, J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr Opin Plant Biol 16, 406–413 (2013).
Yoo, S. D. & Sheen, J. MAPK signaling in plant hormone ethylene signal transduction. Plant signaling & behavior 3, 848–849 (2008).
Wang, F., Wang, C., Yan, Y., Jia, H. & Guo, X. Overexpression of cotton GhMPK11 decreases disease resistance through the gibberellin signaling pathway in transgenic Nicotiana benthamiana. Front Plant Sci 7, 689 (2016).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Soosaar, J. L., Burch-Smith, T. M. & Dinesh-Kumar, S. P. Mechanisms of plant resistance to viruses. Nat Rev Microbiol 3, 789–798 (2005).
Cohn, J. & Martin, G. B. Innate Immunity (Humana Press, 2003).
Haegeman, A., Mantelin, S., Jones, J. T. & Gheysen, G. Functional roles of effectors of plant-parasitic nematodes. Gene 492, 19–31 (2012).
DeYoung, B. J. & Innes, R. W. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7, 1243–1249 (2006).
Richa, K. et al. Functional characterization of novel chitinase genes present in the sheath blight resistance QTL: qSBR11-1 in rice line tetep. Frontiers in plant science 7, 244 (2016).
Boyes, D. C., Nam, J. & Dang, J. L. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc Natl Acad Sci USA 95, 15849–15854 (1998).
Xiao, B. et al. Location of genomic regions contributing to Phytophthora nicotianae resistance in tobacco cultivar ‘Florida 301’. Crop Sci 53, 473–481 (2013).
Chaplin, J. F. Transfer of black shank resistance from Nicotiana plumbaginifolia to flue-cured N.tabacum. Tob. Sci 6, 184–189 (1962).
Sullivan, M. J., Melton, T. A. & Shew, H. D. Fitness of races 0 and 1 of Phytophthora parasitica var. nicotianae. Plant Disease 89, 1220–1228 (2005).
Patel, R. K. & Jain, M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PloS one 7, e30619 (2012).
Sierro, N. et al. The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 5, 3833 (2014).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech 29, 644–652 (2011).
Consortium, T. G. O. The Gene Ontology project in 2008. Nucleic Acids Research 36, D440–D444 (2008).
Ye, J. et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34, W293–W297 (2006).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics 12, 323 (2011).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome biology 11, R106 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate - apractical and powerful approach to multiple testing. Journal of the Royal Statistical Society 57, 289–300 (1995).
Xie, C. et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Research 39, W316–W322 (2011).
Storey, J. D. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 64, 479–498 (2002).
Lalitha, S. Primer Premier 5. Biotech Software & Internet Report (2000).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols 3, 1101–1108 (2008).
Acknowledgements
This work was supported by grants from the YNST (2013HB129), CNTC (110201301006 (JY-06)) and YNTC (2013YN01 and 2016YN23).
Author information
Authors and Affiliations
Contributions
B.G.X. conceived and designed the experiments. J.K.Y. and B.G.X. wrote the paper. J.K.Y. and Z.J.T. performed the experiment and analyzed the data. D.H.F. and X.J.C. collected samples and cultivated bacteria. K.Q.Z. revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, JK., Tong, ZJ., Fang, DH. et al. Transcriptomic profile of tobacco in response to Phytophthora nicotianae infection. Sci Rep 7, 401 (2017). https://doi.org/10.1038/s41598-017-00481-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00481-5
This article is cited by
-
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
Plant and Soil (2024)
-
Potato virus X-mediated constitutive expression of Plutella xylostella PxSDF2L1 gene in Nicotiana benthamiana confers resistance to Phytophthora parasitica var. nicotianae
BMC Plant Biology (2021)
-
Genome-wide identification and characterization of ABA receptor PYL/RCAR gene family reveals evolution and roles in drought stress in Nicotiana tabacum
BMC Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.