Abstract
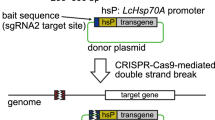
Genomic manipulation is essential to the use of model organisms to understand development, regeneration and adult physiology. The axolotl (Ambystoma mexicanum), a type of salamander, exhibits an unparalleled regenerative capability in a spectrum of complex tissues and organs, and therefore serves as a powerful animal model for dissecting mechanisms of regeneration. We describe here an optimized stepwise protocol to create genetically modified axolotls using the CRISPR–Cas9 system. The protocol, which takes 7–8 weeks to complete, describes generation of targeted gene knockouts and knock-ins and includes site-specific integration of large targeting constructs. The direct use of purified CAS9-NLS (CAS9 containing a C-terminal nuclear localization signal) protein allows the prompt formation of guide RNA (gRNA)–CAS9-NLS ribonucleoprotein (RNP) complexes, which accelerates the creation of double-strand breaks (DSBs) at targeted genomic loci in single-cell-stage axolotl eggs. With this protocol, a substantial number of F0 individuals harboring a homozygous-type frameshift mutation can be obtained, allowing phenotype analysis in this generation. In the presence of targeting constructs, insertions of exogenous genes into targeted axolotl genomic loci can be achieved at efficiencies of up to 15% in a non-homologous end joining (NHEJ) manner. Our protocol bypasses the long generation time of axolotls and allows direct functional analysis in F0 genetically manipulated axolotls. This protocol can be potentially applied to other animal models, especially to organisms with a well-characterized transcriptome but lacking a well-characterized genome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
29 January 2019
In the version of this protocol originally published, the recipe for CAS9 buffer was incorrectly identified as a recipe for sodium acetate solution, and vice versa. These errors have been corrected in the PDF and HTML versions of the paper
References
Voss, S. R., Epperlein, H. H. & Tanaka, E. M. Ambystoma mexicanum, the axolotl: a versatile amphibian model for regeneration, development, and evolution studies. Cold Spring. Harb. Protoc. 2009, pdbemo128 (2009).
Khattak, S. et al. Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-mediated recombination. Nat. Protoc. 9, 529–540 (2014).
Khattak, S. et al. Germline transgenic methods for tracking cells and testing gene function during regeneration in the axolotl. Stem Cell Rep. 1, 90–103 (2013).
Campbell, L. J. et al. Gene expression profile of the regeneration epithelium during axolotl limb regeneration. Dev. Dyn. 240, 1826–1840 (2011).
Putta, S. et al. From biomedicine to natural history research: EST resources for ambystomatid salamanders. BMC Genomics 5, 54 (2004).
Stewart, R. et al. Comparative RNA-seq analysis in the unsequenced axolotl: the oncogene burst highlights early gene expression in the blastema. PLoS Comput. Biol. 9, e1002936 (2013).
Habermann, B. et al. An Ambystoma mexicanum EST sequencing project: analysis of 17,352 expressed sequence tags from embryonic and regenerating blastema cDNA libraries. Genome Biol. 5, R67 (2004).
Bryant, D. M. et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18, 762–776 (2017).
Nowoshilow, S. et al. The axolotl genome and the evolution of key tissue formation regulators. Nature 554, 50–55 (2018).
Voss, S. R. et al. Gene expression during the first 28 days of axolotl limb regeneration I: Experimental design and global analysis of gene expression. Regeneration 2, 120–136 (2015).
Sandoval-Guzman, T. et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174–187 (2014).
Woodcock, M. R. et al. Identification of mutant genes and introgressed tiger salamander DNA in the laboratory axolotl, Ambystoma mexicanum. Sci Rep. 7, 6 (2017).
Whited, J. L., Lehoczky, J. A. & Tabin, C. J. Inducible genetic system for the axolotl. Proc. Natl. Acad. Sci. USA 109, 13662–7 (2012).
Flowers, G. P., Timberlake, A. T., Mclean, K. C., Monaghan, J. R. & Crews, C. M. Highly efficient targeted mutagenesis in axolotl using Cas9 RNA-guided nuclease. Development 141, 2165–71 (2014).
Fei, J. F. et al. CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Rep. 3, 444–459 (2014).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P., Esvelt, K. M. & Church, G. M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Fei, J. F. et al. Tissue- and time-directed electroporation of CAS9 protein–gRNA complexes in vivo yields efficient multigene knockout for studying gene function in regeneration. npj Regen. Med. 1, 16002 (2016).
Fei, J. F. et al. Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proc. Natl. Acad. Sci. USA 114, 12501–12506 (2017).
Smith, J. J. et al. Sal-Site: integrating new and existing ambystomatid salamander research and informational resources. BMC Genomics 6, 181 (2005).
Wang, W. et al. Delivery of Cas9 protein into mouse zygotes through a series of electroporation dramatically increases the efficiency of model creation. J Genet. Genomics 43, 319–327 (2016).
Albadri, S., Del Bene, F. & Revenu, C. Genome editing using CRISPR/Cas9-based knock-in approaches in zebrafish. Methods 121–122, 77–85 (2017).
Burger, A. et al. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 143, 2025–2037 (2016).
Liang, X., Potter, J., Kumar, S., Ravinder, N. & Chesnut, J. D. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J. Biotechnol. 241, 136–146 (2017).
Kim, S., Kim, D., Cho, S. W., Kim, J. & Kim, J. S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Gagnon, J. A. et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186 (2014).
Mircetic, J. et al. Purified Cas9 fusion proteins for advanced genome manipulation. Small Methods 1, 1600052 (2017).
Hu, P., Zhao, X., Zhang, Q., Li, W. & Zu, Y. Comparison of various nuclear localization signal-fused Cas9 proteins and Cas9 mRNA for genome editing in zebrafish. G3 8, 823–831 (2018).
Auer, T. O., Duroure, K., De Cian, A., Concordet, J. P. & Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 24, 142–153 (2014).
He, X. et al. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 44, e85 (2016).
Liang, Z. et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261 (2017).
Svitashev, S., Schwartz, C., Lenderts, B., Young, J. K. & Mark Cigan, A. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 7, 13274 (2016).
Cho, S. W., Lee, J., Carroll, D., Kim, J. S. & Lee, J. Heritable gene knockout in Caenorhabditis elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics 195, 1177–1180 (2013).
Paix, A., Folkmann, A., Rasoloson, D. & Seydoux, G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics 201, 47–54 (2015).
Lee, J. S. et al. RNA-guided genome editing in Drosophila with the purified Cas9 protein. G3 4, 1291–5 (2014).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55 (2013).
Kuo, T. H. et al. TALEN-mediated gene editing of the thrombospondin-1 locus in axolotl. Regeneration 2, 37–43 (2015).
Grabher, C. & Wittbrodt, J. Meganuclease and transposon mediated transgenesis in medaka. Genome Biol. 8, S10 (2007).
Saigou, Y., Kamimura, Y., Inoue, M., Kondoh, H. & Uchikawa, M. Regulation of Sox2 in the pre-placodal cephalic ectoderm and central nervous system by enhancer N-4. Dev. Growth Differ. 52, 397–408 (2010).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Kim, E. et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 8, 14500 (2017).
Muller, M. et al. Streptococcus thermophilus CRISPR-Cas9 systems enable specific editing of the human genome. Mol. Ther. 24, 636–644 (2016).
Lee, C. M., Cradick, T. J. & Bao, G. The Neisseria meningitidis CRISPR-Cas9 system enables specific genome editing in mammalian cells. Mol. Ther. 24, 645–654 (2016).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015).
Kleinstiver, B. P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Kleinstiver, B. P. et al. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 33, 1293–1298 (2015).
Hu, J. H. et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 556, 57–63 (2018).
Li, J. et al. Intron targeting-mediated and endogenous gene integrity-maintaining knockin in zebrafish using the CRISPR/Cas9 system. Cell Res. 25, 634–637 (2015).
Hisano, Y. et al. Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Sci. Rep. 5, 8841 (2015).
Sander, J. D. & Joung, J. K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Kotani, H., Taimatsu, K., Ohga, R., Ota, S. & Kawahara, A. Efficient multiple genome modifications induced by the crRNAs, tracrRNA and Cas9 protein complex in zebrafish. PLoS ONE 10, e0128319 (2015).
Sung, Y. H. et al. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 24, 125–131 (2014).
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M. & Joung, J. K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2014).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–866 (2013).
Kochetov, A. V. Alternative translation start sites and hidden coding potential of eukaryotic mRNAs. BioEssays 30, 683–691 (2008).
Harrison, M. M., Jenkins, B. V., O’Connor-Giles, K. M. & Wildonger, J. A CRISPR view of development. Genes Dev. 28, 1859–1872 (2014).
Elewa, A. et al. Reading and editing the Pleurodeles waltl genome reveals novel features of tetrapod regeneration. Nat. Commun. 8, 2286 (2017).
Bell, C. C., Magor, G. W., Gillinder, K. R. & Perkins, A. C. A high-throughput screening strategy for detecting CRISPR-Cas9 induced mutations using next-generation sequencing. BMC Genomics 15, 1002 (2014).
Sentmanat, M. F., Peters, S. T., Florian, C. P., Connelly, J. P. & Pruett-Miller, S. M. A survey of validation strategies for CRISPR-Cas9 editing. Sci Rep. 8, 888 (2018).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
Kim, J. M., Kim, D., Kim, S. & Kim, J. S. Genotyping with CRISPR-Cas-derived RNA-guided endonucleases. Nat. Commun. 5, 3157 (2014).
Vouillot, L., Thelie, A. & Pollet, N. Comparison of T7E1 and surveyor mismatch cleavage assays to detect mutations triggered by engineered nucleases. G3 5, 407–415 (2015).
Qiu, P. et al. Mutation detection using surveyor nuclease. Biotechniques 36, 702–707 (2004).
Ota, S. et al. Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells 18, 450–458 (2013).
Shigeta, M. et al. Rapid and efficient analysis of gene function using CRISPR-Cas9 in Xenopus tropicalis founders. Genes Cells 21, 755–771 (2016).
Zhu, X. et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 4, 6420 (2014).
Dahlem, T. J. et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8, e1002861 (2012).
Samarut, E., Lissouba, A. & Drapeau, P. A simplified method for identifying early CRISPR-induced indels in zebrafish embryos using high resolution melting analysis. BMC Genomics 17, 547 (2016).
Guschin, D. Y. et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol. Biol. 649, 247–256 (2010).
Gansauge, M. T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8, 737–748 (2013).
Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 6, e18556 (2011).
Pelletier, J. & Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334, 320–355 (1988).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Wilton, S. Direct sequencing of PCR products. eLS https://doi.org/10.1038/npg.els.0003769 (2002).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Brocal, I. et al. Efficient identification of CRISPR/Cas9-induced insertions/deletions by direct germline screening in zebrafish. BMC Genomics 17, 259 (2016).
Irion, U., Krauss, J. & Nusslein-Volhard, C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141, 4827–4330 (2014).
Armstrong, G. A. et al. Homology directed knockin of point mutations in the zebrafish tardbp and fus genes in ALS using the CRISPR/Cas9 system. PLoS ONE 11, e0150188 (2016).
Nakayama, T. et al. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis 51, 835–843 (2013).
Pinello, L. et al. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat. Biotechnol. 34, 695–697 (2016).
Acknowledgements
We thank M. Schuez for outstanding technical support, and B. Gruhl, A. Wagner and S. Kaudel for professional axolotl care. This research was supported by German Research Foundation (DFG) grants DFG 274/3-2 (to E.M.T.), DFG 274/3-3 (to E.M.T.), and DFG 274/2-3/SFB655 (from Cells into Tissues; to E.M.T.); a European Research Council Advanced Investigator grant (294324; to E.M.T.), a Human Frontier Science Program grant (RGP0016/2010; to E.M.T.), Central Funds from the DFG Research Center for Regenerative Therapies Dresden (FZ111; to E.M.T.), National Nature Science Foundation of China (NSFC) grant 31771611 (to J.-F.F.), Research Starting grants from South China Normal University (S82111 and 8S0109; to J.-F.F.) and a China Postdoctoral Science Foundation grant (2018M633067; to W.P.-K.L.).
Author information
Authors and Affiliations
Contributions
J.-F.F., D.K. and E.M.T. conceived and designed the experiments. J.-F.F., D.K., P.M., T.G., Y.T. and S.N. performed the experiments. W.P.-K.L. and S.K. contributed to the data analysis. J.-F.F., W.P.-K.L. and E.M.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Nowoshilow, S. et al. Nature. 554, 50–55 (2018): https://doi.org/10.1038/nature25458
Fei, J.-F. et al. Proc. Natl. Acad. Sci. USA 114, 12501–12506 (2017): https://doi.org/10.1073/pnas.1706855114
Fei, J.-F. et al. Stem Cell Rep. 3, P444–P459 (2014): https://doi.org/10.1016/j.stemcr.2014.06.018
Integrated supplementary information
Supplementary Figure 1 Recommended plate setup for NGS.
The recommended combinations of P5-indexes-forward primers (in total 12, #1–12) and P7-indexes-reverse primers (in total 8, A-H) on a 96-well plate for NGS.
Supplementary Figure 2 Examples of NGS PCR on individual F0 CRISPR axolotls injected with gRNA-CAS9-NLS RNP complexes.
Examples of NGS-PCR1 (a) and NGS-PCR2 (b) on individual F0 CRISPR axolotls from two independent genomic loci:, Tyrosinase and Yap1. Red stars indicate the PCR products carrying large indels, which run faster on the gel than the PCR products amplified from unmodified alleles or those carrying small indels. All products from NGS-PCR2 should be sequenced, since it is impossible to determine the genotype solely by the gel picture (PCR products harbour small indels are indistinguishable from the wild-type PCR product).
Supplementary Figure 3 Examples of NGS analysis of three individual F0 Tyrosinase (Tyr) CRISPR axolotls.
(a) An animal harbouring only two types of modifications (homozygous-type) at the Tyr-gRNA targeted locus. It likely represents modifications which occurred separately on the paternal and maternal alleles in a single cell stage axolotl eggs. (b) A mosaic animal harbouring 100% modified Tyrosinase locus, with many types of indels. (c) An animal with incompletely modified Tyrosinse locus; a proportion (approximately 16%) of the alleles are unmodified. Tyr-gRNA target (red); PAM sequence (blue); characters in squares indicate single nucleotide substitutions (SNS), which appear only at very low percentage and likely represent mutations generated during PCR. Therefore, we categorize the same type of modification with or without SNS as one group.
Supplementary Figure 4 Genomic PCR and Sanger sequence analysis of individual F0 Pax7:Cherry knock-in axolotls.
(a) Genomic PCR of the Pax7 locus of individual F0 Pax7:Cherry knock-in axolotls (animal #1–4) and the negative control (Ctr, un-injected sibling of the F0 knock-in axolotls). Upon NHEJ-mediated insertion of the Cherry reporter gene into the Pax7 locus, approximately 2.8 kb PCR products are amplified using the primer pair Pax7-fw and pA-rev (Fig. 7 and Table 4). (b) Sequence analysis reveals that in-frame scars (5’ integration junctions, animal #1: 15nt deletion; animal #2: 9nt deletion; animal #3: 9nt and 7nt deletions; animal #4: 6nt deletion) are present in all F0 Pax7:Cherry animals. Note: the in-frame integration junctions in the limb (limb junct) and tail (tail junct) from the same individuals are identical in three different knock-in axolotls, animal #1, #2 and #4. All animal experiments were carried out according to relevant Institutional and National regulations. Adapted with permission from Fei, J.F. et al. Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proc. Natl. Acad. Sci. USA 114, 12501–12506 (2017).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1 and 2 and Supplementary Methods 1 and 2
Rights and permissions
About this article
Cite this article
Fei, JF., Lou, W.PK., Knapp, D. et al. Application and optimization of CRISPR–Cas9-mediated genome engineering in axolotl (Ambystoma mexicanum). Nat Protoc 13, 2908–2943 (2018). https://doi.org/10.1038/s41596-018-0071-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0071-0
This article is cited by
-
A perspective on neuroethology: what the past teaches us about the future of neuroethology
Journal of Comparative Physiology A (2024)
-
The specialist in regeneration—the Axolotl—a suitable model to study bone healing?
npj Regenerative Medicine (2022)
-
Tig1 regulates proximo-distal identity during salamander limb regeneration
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.