Abstract
The neurodegenerative disease spinal muscular atrophy (SMA) is caused by deficiency in the survival motor neuron (SMN) protein. Currently approved SMA treatments aim to restore SMN, but the potential for SMN expression beyond physiological levels is a unique feature of adeno-associated virus serotype 9 (AAV9)-SMN gene therapy. Here, we show that long-term AAV9-mediated SMN overexpression in mouse models induces dose-dependent, late-onset motor dysfunction associated with loss of proprioceptive synapses and neurodegeneration. Mechanistically, aggregation of overexpressed SMN in the cytoplasm of motor circuit neurons sequesters components of small nuclear ribonucleoproteins, leading to splicing dysregulation and widespread transcriptome abnormalities with prominent signatures of neuroinflammation and the innate immune response. Thus, long-term SMN overexpression interferes with RNA regulation and triggers SMA-like pathogenic events through toxic gain-of-function mechanisms. These unanticipated, SMN-dependent and neuron-specific liabilities warrant caution on the long-term safety of treating individuals with SMA with AAV9-SMN and the risks of uncontrolled protein expression by gene therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in this paper or the Supplementary Information. Sequencing data have been deposited in the Gene Expression Omnibus database under accession number GSE149391. Source data are provided with this paper. Any other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Burghes, A. H. M. & Beattie, C. E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10, 597–609 (2009).
Tisdale, S. & Pellizzoni, L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 35, 8691–8700 (2015).
Lefebvre, S. et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995).
Lorson, C. L., Hahnen, E., Androphy, E. J. & Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl Acad. Sci. USA 96, 6307–6311 (1999).
Hua, Y. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123–126 (2011).
Porensky, P. N. et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 21, 1625–1638 (2012).
Naryshkin, N. A. et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345, 688–693 (2014).
Palacino, J. et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 11, 511–517 (2015).
Foust, K. D. et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 28, 271–274 (2010).
Passini, M. A. et al. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest. 120, 1253–1264 (2010).
Dominguez, E. et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 20, 681–693 (2011).
Valori, C. F. et al. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl. Med. 2, 35ra42 (2010).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378, 625–635 (2018).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Al-Zaidy, S. et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr. Pulmonol. 54, 179–185 (2019).
Dhillon, S. Risdiplam: first approval. Drugs 80, 1853–1858 (2020).
Mercuri, E., Pera, M. C., Scoto, M., Finkel, R. & Muntoni, F. Spinal muscular atrophy—insights and challenges in the treatment era. Nat. Rev. Neurol. https://doi.org/10.1038/s41582-020-00413-4 (2020).
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298 (2018).
National Library of Medicine. Study of intrathecal administration of onasemnogene abeparvovec-xioi for spinal muscular atrophy (STRONG). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03381729 (2017).
European Medicines Agency. Zolgensma: European public assessment report. https://www.ema.europa.eu/en/documents/assessment-report/zolgensma-epar-public-assessment-report_en.pdf (2020).
Passini, M. A. et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum. Gene Ther. 25, 619–630 (2014).
Gray, S. J. & Samulski, R. Vector design and considerations for CNS applications. in Gene Vector Design and Application to Treat Nervous System Disorders (ed. Glorioso. J.) 7–15 (Society for Neuroscience, 2011).
Taylor, M. D. et al. Postnatal regulation of limb proprioception by muscle-derived neurotrophin-3. J. Comp. Neurol. 432, 244–258 (2001).
Simon, C. M. et al. Stasimon contributes to the loss of sensory synapses and motor neuron death in a mouse model of spinal muscular atrophy. Cell Rep. 29, 3885–3901 (2019).
Mentis, G. Z. et al. Early functional impairment of sensorimotor connectivity in a mouse model of spinal muscular atrophy. Neuron 69, 453–467 (2011).
Fletcher, E. V. et al. Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat. Neurosci. 20, 905–916 (2017).
Simon, C. M. et al. Converging mechanisms of p53 activation drive motor neuron degeneration in spinal muscular atrophy. Cell Rep. 21, 3767–3780 (2017).
Li, D. K., Tisdale, S., Lotti, F. & Pellizzoni, L. SMN control of RNP assembly: from post-transcriptional gene regulation to motor neuron disease. Semin. Cell Dev. Biol. 32, 22–29 (2014).
Ruggiu, M. et al. A role for SMN exon 7 splicing in the selective vulnerability of motor neurons in spinal muscular atrophy. Mol. Cell. Biol. 32, 126–138 (2012).
Van Alstyne, M. et al. Dysregulation of Mdm2 and Mdm4 alternative splicing underlies motor neuron death in spinal muscular atrophy. Genes Dev. 32, 1045–1059 (2018).
Saltzman, A. L., Pan, Q. & Blencowe, B. J. Regulation of alternative splicing by the core spliceosomal machinery. Genes Dev. 25, 373–384 (2011).
Tenner, A. J., Stevens, B. & Woodruff, T. M. New tricks for an ancient system: physiological and pathological roles of complement in the CNS. Mol. Immunol. 102, 3–13 (2018).
Vukojicic, A. et al. The classical complement pathway mediates microglia-dependent remodeling of spinal motor circuits during development and in SMA. Cell Rep. 29, 3087–3100 (2019).
Lucin, K. M. & Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122 (2009).
Sleigh, J. N. et al. Chondrolectin affects cell survival and neuronal outgrowth in in vitro and in vivo models of spinal muscular atrophy. Hum. Mol. Genet. 23, 855–869 (2014).
Nussbacher, J. K., Tabet, R., Yeo, G. W. & Lagier-Tourenne, C. Disruption of RNA metabolism in neurological diseases and emerging therapeutic interventions. Neuron 102, 294–320 (2019).
Monani, U. R. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 9, 333–339 (2000).
Gavrilina, T. O. et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum. Mol. Genet. 17, 1063–1075 (2008).
Lorson, C. L. et al. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 19, 63–66 (1998).
Pellizzoni, L., Charroux, B. & Dreyfuss, G. SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc. Natl Acad. Sci. USA 96, 11167–11172 (1999).
Bühler, D., Raker, V., Lührmann, R. & Fischer, U. Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet. 8, 2351–2357 (1999).
U.S. Food and Drug Administration. AveXis. Zolgensma (onasemnogene abeparvovec-xioi) package insert. https://www.fda.gov/vaccines-blood-biologics/zolgensma (2019).
Hordeaux, J. et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 26, 664–668 (2018).
Schuster, D. J. et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front. Neuroanat. 8, 42 (2014).
DiDonato, C. J. et al. Cloning, characterization and copy number of the murine survival motor neuron gene homolog of the spinal muscular atrophy-determining gene. Genome Res. 7, 339–352 (1997).
Lorson, M. A., Spate, L. D., Prather, R. S. & Lorson, C. L. Identification and characterization of the porcine (Sus scrofa) survival motor neuron (SMN1) gene: an animal model for therapeutic studies. Dev. Dyn. 237, 2268–2278 (2008).
Wu, J., Anczuków, O., Krainer, A. R., Zhang, M. Q. & Zhang, C. OLego: fast and sensitive mapping of spliced mRNA-seq reads using small seeds. Nucleic Acids Res. 41, 5149–5163 (2013).
Yan, Q. et al. Systematic discovery of regulated and conserved alternative exons in the mammalian brain reveals NMD modulating chromatin regulators. Proc. Natl Acad. Sci. USA 112, 3445–3450 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Robinson, M. D., Mccarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Acknowledgements
We are grateful to S. Jaffrey for comments and critical reading of the manuscript. This work was supported by NIH grants R01NS102451 (to L.P.), R21NS099977 (to L.P.), R01NS078375 (to G.Z.M.) and R01AA027079 (to G.Z.M.). High-performance computation was supported by NIH grants S10OD012351 and S10OD021764. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA013696 and used the Genomics and High-Throughput Screening Shared Resource.
Author information
Authors and Affiliations
Contributions
L.P. designed and supervised the study. M.V.A. performed the experiments and analyzed the data except as noted below. I.T. and E.W. contributed to behavioral studies. N.D. performed electrophysiological studies and data analysis. Y.R. and C.Z. carried out bioinformatic analysis of RNA-seq data. L.S.S. provided AAV9 vectors. G.Z.M. contributed to the design, analysis and interpretation of functional studies. M.V.A. and L.P. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Long-term AAV9-mediated SMN overexpression decreases sensory-motor synaptic transmission of proprioceptive neurons.
a-c, Representative EMG recordings from the footpad of uninjected (a) and either AAV9-GFP (5) (b) or AAV9-SMN (5) (c) treated WT mice at P300 using a stimulation frequency of 0.1 Hz. This analysis captures both proprioceptive synaptic function (H-reflex) and motor neuron function (M-response), and the H/M ratio can determine a preferential deficit in either response. Arrows point to the stimulus artifact. d, Amplitude of the M-response from the same groups as shown in (a-c). The box-and-whiskers graph shows the median, interquartile range, minimum and maximum (n = 8 animals). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (ns) no significance. P = 0.9741, F2, 21 = 0.02629. e, Amplitude of H-reflex from the same groups as shown in (a-c). The box-and-whiskers graph shows the median, interquartile range, minimum and maximum (n = 8 animals). Statistics were performed with one-way ANOVA with Tukey’s post-hoc test. (*) P < 0.05. WT vs SMN(5): P = 0.0420, q = 3.684, d.f.=21. f, Ratio of H-reflex and M-response from the same groups as shown in (a-c). The box-and-whiskers graph shows the median, interquartile range, minimum and maximum (n = 8 animals). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (*) P < 0.05. WT vs SMN(5): P = 0.0103, q = 4.592, df=21. g, Repetitive nerve stimulation at different frequencies from 0.1 to 50 Hz does not change the amplitude of the M-response recorded from the footpad of uninjected and either AAV9-GFP (5) or AAV9-SMN (5) treated WT mice at P300. The box-and-whiskers graph shows the median, interquartile range, minimum and maximum from the following number of animals: WT (0.1-10 Hz n = 8; 20-50 Hz n = 6); WT + AAV-GFP(5) (n = 8); WT + AAV-SMN(5) (0.1-10 Hz n = 8; 20 Hz n = 7; 50 Hz n = 6). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (ns) no significance. WT: P = 0.9938, F4, 31 = 0.05630; WT + AAV-GFP(5): P = 0.6199, F4, 35 = 0.06660; WT + AAV-SMN(5): P = 0.5344, F4, 32 = 0.07997.
Extended Data Fig. 2 Effects of AAV9-SMN on motor neuron survival, C-boutons and NMJ innervation in WT mice.
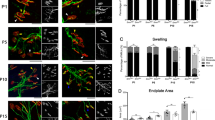
a, ChAT immunostaining of L5 spinal segments from uninjected, AAV9-GFP (5) and AAV9-SMN (5) injected WT mice at P150. Scale bar=100 µm. b, Soma size of MNs from the same groups as in (a) at P150. The violin plot shows the median (solid line) and interquartile range (dotted lines) from the following number of MNs (WT, n = 227 neurons; WT + GFP(5), n = 234 neurons; WT + SMN(5), n = 234 neurons) from 3 animals per group. Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (ns) no significance. P = 0.3083, F3, 449 = 1.180. c, Percentage of the number of L5 LMC MNs per 75 µm section relative to WT in the same groups as in (a) at P150. The box-and-whiskers graph shows the median, interquartile range, minimum and maximum from the following number of sections (WT, n = 36 sections; WT + GFP(5), n = 31 sections; WT + SMN(5), n = 43 sections) from 3 animals per group. Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (*) P < 0.05; (**) P < 0.01. WT vs SMN(5): P = 0.0284, q = 3.678, df=107; GFP(5) vs SMN(5): P = 0.0064, q = 4.420, df=107. d, ChAT immunostaining of L5 LMC MNs from uninjected, AAV9-GFP (5) and AAV9-SMN (5 and 10) injected WT mice at P300. Scale bar=25 µm. e, Total number of ChAT+ C-boutons on L5 LMC MNs from the same groups as in (d) at P150 and P300. The box-and-whiskers graph shows the median, interquartile range, minimum and maximum from the following numbers of neurons and animals at P150 (WT, n = 39 neurons, n = 3 animals; WT + GFP(5), n = 37 neurons, n = 3 animals; WT + SMN(5), n = 49 neurons, n = 3 animals) and P300 (WT, n = 28 neurons, n = 3 animals; WT + GFP(5), n = 31 neurons, n = 3 animals; WT + SMN(5), n = 36 neurons, n = 3 animals; WT + SMN(10), n = 14 neurons, n = 4 animals). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (ns) no significance. For MNs at P150, P = 0.9855, F2, 122 = 0.01456. For MNs at P300, P = 0.0651, F3, 105 = 2.481. f, NMJ immunostaining of EDL muscles with Neurofilament-M (NF-M), Synaptophysin (SYP) and bungarotoxin (BTX) from the same groups as in (a) at P300. Scale bar=25 µm. g, Percentage of innervated, partially innervated and denervated NMJs in the EDL muscle from the same groups as in (a) at P150 and P300. Data represent mean and SEM (n = 3 animals). Statistics were performed with two-way ANOVA with Tukey’s post hoc test. (*) P < 0.05. For innervated NMJs: SMN(5) P150 vs SMN(5) P300: P = 0.0346, q = 4.588, df=42; SMN(5) P150 vs SMN(10) P300: P = 0.0317, q = 4.636, df=42.
Extended Data Fig. 3 Effects of AAV9-SMN on motor neuron survival, soma size and NMJ innervation in SMA mice.
a, ChAT immunostaining of L2 MNs from untreated WT (SMNΔ7) mice and either AAV9-GFP (5) or AAV9-SMN (5) treated SMA mice at the indicated times. Scale bars=75 µm (P11) and 100 µm (P190). b, NMJ immunostaining with Neurofilament-M (NF-M), Synaptophysin (SYP) and bungarotoxin (BTX) of QL muscles from the same groups as in (a). Scale bar=25 µm (P11) and 50 µm (P190). c, Soma size of MNs from the same groups as in (a) at P11 and P190. The violin plot shows the median (solid line) and interquartile range (dotted lines) from the following number of MNs at P11 (WT (SMNΔ7), n = 230 neurons; SMA + GFP(5), n = 185 neurons; SMA + SMN(5), n = 175 neurons) and P190 (WT (SMNΔ7), n = 231 neurons; SMA + SMN(5), n = 164 neurons) from 3 animals per group. Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (**) P < 0.01; (****) P < 0.0001. WT (SMNΔ7) P11 vs SMA + GFP(5) P11: P < 0.0001, q = 7.696, df=980; SMA + GFP(5) P11 vs SMA + SMN(5) P11: P = < 0.0001, q = 7.714, df=980; WT (SMNΔ7) P190 vs SMA + SMN(5) P190: P = 0.0058, q = 4.841, df=980. d, Percentage of the number of MNs per 75 µm section relative to WT (SMNΔ7) in the same groups as in (a) at P11 and P190. The box-and-whiskers graph shows the median, interquartile range, minimum and maximum from the following number of sections and animals at P11 (WT (SMNΔ7), n = 48 sections, n = 3 animals; SMA + GFP(5), n = 58 sections, n = 6 animals; SMA + SMN(5), n = 56 sections n = 6 animals) and P190 (WT (SMNΔ7), n = 42 sections, n = 3 animals; SMA + SMN(5), n = 41 sections, n = 3 animals). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (**) P < 0.01; (***) P < 0.001; (ns) no significance. WT (SMNΔ7) P11 vs SMA + GFP(5) P11: P < 0.0001, q = 13.58, df=241; WT (SMNΔ7) P11 vs SMA + SMN(5) P11: P < 0.0001, q = 8.359, df=241; SMA + GFP(5) P11 vs SMA + SMN(5) P11: P = 0.0018, q = 5.364, df=241; WT (SMNΔ7) P190 vs SMA + SMN(5) P190: P < 0.0001, q = 8.108, df=241; SMA + SMN(5) P11 vs SMA + SMN(5) P190: P = 0.9927, q = 0.6110, df=241. e, Percentage of innervated, partially innervated and denervated NMJs relative to WT (SMNΔ7) in the QL muscle from the same groups as in (b) at P11 and P190. Data represent mean and SEM (n = 3 animals). Statistics were performed with two-way ANOVA with Tukey’s post hoc test. (****) P < 0.0001; (ns) no significance. For innervated NMJs: WT (SMNΔ7) P11 vs SMA + GFP(5) P11: P < 0.0001, q = 10.72, df=30; SMA + GFP(5) P11 vs SMA + SMN(5) P11: P < 0.0001, q = 7.789, df=30; SMA + SMN(5) P11 vs SMA + SMN(5) P190: P = 0.9999, q = 0.2233, df=30. f, Total number of PV+ PNs in L2 DRGs from untreated WT (SMNΔ7) mice and AAV9-SMN (5) treated SMA mice at P190. Data represent mean and SEM (n = 3 DRGs from n = 3 animals). Statistics were performed with unpaired Student’s t-test. (ns) no significance. P = 0.9106, t = 0.1196, df=4.
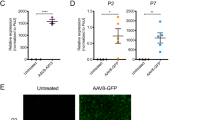
Extended Data Fig. 4 Efficiency of AAV9 transduction and long-term transgene expression in tissues of WT mice.
a, Percentage of GFP+ L5 LMC MNs from uninjected and AAV9-GFP (5) injected WT mice at P300. Data represent mean and SEM (n = 3 animals). b, Percentage of GFP+ PNs in L5 DRGs from the same groups as in (a) at P300. Data represent mean and SEM (n = 3 animals). c, RT-qPCR analysis of GFP mRNA in spinal cord, DRG and liver from WT mice injected with AAV9-GFP (5) at P11 and P300. Data represent mean and SEM (n = 3 animals) normalized to Gapdh mRNA and expressed relative to levels at P11 (set as 1). d, RT-qPCR analysis of full-length human SMN (hSMN-FL) mRNA in spinal cord, DRG and liver from WT mice injected with AAV9-SMN (5) at P11 and P300. Data represent mean and SEM (n = 3 animals) normalized to Gapdh mRNA and expressed relative to levels at P11 (set as 1).
Extended Data Fig. 5 AAV9-SMN leads to persistent SMN overexpression in the spinal cord and DRGs but not the liver of SMA mice.
a-c, RT-qPCR analysis of hSMN-FL mRNA levels in spinal cord (a), DRG (b) and liver (c) of uninjected WT (SMNΔ7) mice and SMA mice injected with either AAV9-GFP (5) or AAV9-SMN (5) at the indicated times. Data represent mean and SEM (n = 3 animals) normalized to Gapdh mRNA and expressed relative to levels in WT (SMNΔ7) mice (set as 1). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (*) P < 0.05; (****) P < 0.0001; (ns) no significance. For spinal cord: SMA + SMN(5) P11 vs SMA + SMN(5) P190: P = 0.5887, q = 2.111, df=10. For DRG: SMA + SMN(5) P11 vs SMA + SMN(5) P190: P = 0.0396, q = 4.866, df=10. For liver: SMA + SMN(5) P11 vs SMA + SMN(5) P190: P < 0.0001, q = 16.28, df=10. d-e, Western blot analysis of spinal cord (d) and liver (e) from the same groups as in (a). SMN expression was analyzed with antibodies that specifically detect only human SMN (hSMN) or both human and mouse SMN (pan-SMN). Cropped images are shown. f, Quantification of total SMN protein levels from the Western blot analysis in (d). Data represent mean and SEM (n = 3 animals) normalized to Gapdh and expressed relative to levels in WT (SMNΔ7) mice (set as 1). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (****) P < 0.0001; (***) P < 0.001. WT (SMNΔ7) P11 vs SMA + GFP(5) P11: P < 0.0001, q = 24.80, df=10; WT (SMNΔ7) P11 vs WT (SMNΔ7) P190: P = 0.0001, q = 11.12, df=10; WT (SMNΔ7) P11 vs SMA + SMN(5) P190: P = 0.0003, q = 9.975, df=10; SMA + SMN(5) P11 vs SMA + SMN(5) P190: P < 0.0001, q = 11.23, df=10. g, Quantification of human SMN protein levels from the Western blot analysis in (d). Data represent mean and SEM (n = 3 animals) normalized to Gapdh and expressed relative to levels in WT (SMNΔ7) mice (set as 1). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (****) P < 0.0001; (***) P < 0.001; (**) P < 0.01; (*) P < 0.05. WT (SMNΔ7) P11 vs SMA + GFP(5) P11: P = 0.0165, q = 5.671, df=10; WT (SMNΔ7) P11 vs SMN(5) P11: P < 0.0001, q = 25.08, df=10; WT (SMNΔ7) P11 vs SMA + SMN(5) P190: P = 0.0009, q = 8.513, df=10. SMA + SMN(5) P11 vs SMA + SMN(5) P190: P < 0.0001, q = 16.57, df=10. h, Quantification of total SMN protein levels from the Western blot analysis in (e). Data represent mean and SEM (n = 3 animals) normalized to Gapdh and expressed relative to levels in WT (SMNΔ7) mice (set as 1). Statistics were performed with one-way ANOVA with Tukey’s post hoc test. (****) P < 0.0001. WT (SMNΔ7) P11 vs SMA + SMN(5) P11: P < 0.0001, q = 13.08, df=10; SMA + SMN(5) P11 vs SMA + SMN(5) P190: P < 0.0001, q = 15.56, df=10.
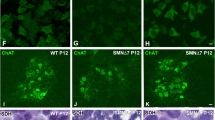
Extended Data Fig. 6 AAV9-SMN drives cytoplasmic aggregation of overexpressed SMN in motor circuit neurons of mouse models.
a, ChAT and SMN immunostaining of L5 LMC MNs from uninjected and either AAV9-GFP (5) or AAV9-SMN (5) injected WT mice at P11 and P150. Scale bar=10 µm. b, Percentage of L5 LMC MNs with SMN aggregates in the same groups as in (a) at P300. Data represent mean and SEM (n = 3 animals). c, PV and SMN immunostaining of L5 DRG PNs from uninjected and either AAV9-GFP (5) or AAV9-SMN (5) injected WT mice at P11 and P150. Scale bar=10 µm. d, Percentage of L5 DRG PNs with SMN aggregates in the same groups as in (c) at P300. Data represent mean and SEM (n = 3 animals). e, ChAT and SMN immunostaining of L2 MNs from WT (SMNΔ7) mice and either AAV9-GFP (5) or AAV9-SMN (5) injected SMA mice at the indicated time points. Scale bar=10 µm. f, Percentage of L2 MNs with SMN aggregates in the same groups as in (e) at P190. Data represent mean and SEM (n = 3 animals). g, PV and SMN immunostaining of L2 DRG PNs from the same groups as in (e). Scale bar=10 µm. h, Percentage of L2 PNs with SMN aggregates in the same groups as in (g) at P190. Data represent mean and SEM (n = 3 animals).
Extended Data Fig. 7 AAV9-mediated SMN overexpression induces cytoplasmic aggregation of SmB in motor circuit neurons of mouse models.
a, ChAT and SmB immunostaining of L5 LMC MNs from uninjected and either AAV9-GFP (5) or AAV9-SMN (5) injected WT mice at P11 and P150. Scale bar=10 µm. b, PV and SmB immunostaining of L5 DRG PNs from the same groups as in (a). Scale bar=10 µm. c, ChAT and SmB immunostaining of L2 MNs from WT (SMNΔ7) mice and either AAV9-GFP (5) or AAV9-SMN (5) injected SMA mice at the indicated time points. Scale bar=10 µm. d, PV and SmB immunostaining of L2 PNs from the same groups as in (c). Scale bar=10 µm.
Extended Data Fig. 8 Immunohistochemical analysis of cytoplasmic SMN aggregates in motor neurons.
a-d, Immunostaining of L5 LMC MNs from WT mice injected with AAV9-SMN (5) at P300 with DAPI, ChAT, SMN and RNA-binding proteins KSRP (a), FUS (b), hnRNPA1 (c) or HuR (d) as indicated. Scale bar=5 µm. e-f, Immunostaining with DAPI, ChAT and RNA transport factors Ran (e) and Crm1 (f) in the same MNs as in (a). Scale bar=5 µm. g-h, Immunostaining with DAPI, ChAT, SMN and either GW182 to label P-bodies (g) or TIA-1 to label stress granules (h) in the same MNs as in (a). Scale bar=5 µm. i-j, Immunostaining with DAPI, ChAT, SMN and either ubiquitin (i) or the autophagy marker LC3B (j) in the same MNs as in (a). Scale bar=5 µm.
Extended Data Fig. 9 Viral-mediated overexpression of human SMN induces cytoplasmic aggregation and sequestration of SmB in human cells.
HeLa cells were untreated (mock) or transduced with lentivirus expressing either GFP (Lenti-GFP) or human SMN (Lenti-SMN). Immunostaining with DAPI, GFP, SMN and SmB was performed five days post transduction. Scale bar=10 µm.
Extended Data Fig. 10 Model of the toxic effects of long-term AAV9-mediated SMN overexpression in neurons.
Schematic depicting the gain of toxic function mechanisms induced by long-term overexpression of SMN in the sensory-motor circuit of mouse models (see text for further details).
Supplementary information
Supplementary Tables 1–5
Supplementary tables and descriptive legends.
Supplementary Video 1
Clasping phenotype of WT mice injected with AAV9-SMN.
Supplementary Video 2
Hind-limb paralysis of a P300 WT mouse injected with a high dose (1 × 1011 vg g−1) of AAV9-SMN.
Source data
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Van Alstyne, M., Tattoli, I., Delestrée, N. et al. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat Neurosci 24, 930–940 (2021). https://doi.org/10.1038/s41593-021-00827-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00827-3
This article is cited by
-
AAV-delivered muscone-induced transgene system for treating chronic diseases in mice via inhalation
Nature Communications (2024)
-
Promoting expression in gene therapy: more is not always better
EMBO Molecular Medicine (2024)
-
Programmable RNA base editing via targeted modifications
Nature Chemical Biology (2024)
-
Improved gene therapy for spinal muscular atrophy in mice using codon-optimized hSMN1 transgene and hSMN1 gene-derived promotor
EMBO Molecular Medicine (2024)
-
The era of cryptic exons: implications for ALS-FTD
Molecular Neurodegeneration (2023)