Abstract
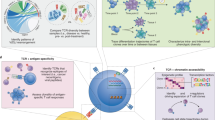
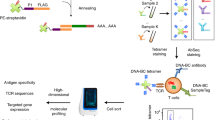
T cells respond to threats in an antigen-specific manner using T cell receptors (TCRs) that recognize short peptide antigens presented on major histocompatibility complex (MHC) proteins. The TCR–peptide-MHC interaction mediated between a T cell and its target cell dictates its function and thereby influences its role in disease. A lack of approaches for antigen discovery has limited the fundamental understanding of the antigenic landscape of the overall T cell response. Recent advances in high-throughput sequencing, mass cytometry, microfluidics and computational biology have led to a surge in approaches to address the challenge of T cell antigen discovery. Here, we summarize the scope of this challenge, discuss in depth the recent exciting work and highlight the outstanding questions and remaining technical hurdles in this field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davis, M. M. & Bjorkman, P. J. T-cell antigen receptor genes and T-cell recognition. Nature 334, 395–402 (1988).
La Gruta, N. L., Gras, S., Daley, S. R., Thomas, P. G. & Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 18, 467–478 (2018).
Robins, H. S. et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci. Transl. Med. 2, 47ra64 (2010).
Paucek, R. D., Baltimore, D. & Li, G. The cellular immunotherapy revolution: arming the immune system for precision therapy. Trends Immunol. 40, 292–309 (2019).
Germain, R. N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 (2002).
Godfrey, D. I., Le Nours, J., Andrews, D. M., Uldrich, A. P. & Rossjohn, J. Unconventional T cell targets for cancer immunotherapy. Immunity 48, 453–473 (2018).
Rock, K. L., Reits, E. & Neefjes, J. Present yourself! By MHC class I and MHC class II molecules. Trends Immunol. 37, 724–737 (2016).
Neefjes, J., Jongsma, M. L., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
Reinherz, E. L. & Wang, J. H. Codification of bidentate pMHC interaction with TCR and its co-receptor. Trends Immunol. 36, 300–306 (2015).
Sewell, A. K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 669–677 (2012).
Nikolich-Zugich, J., Slifka, M. K. & Messaoudi, I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 4, 123–132 (2004).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19, 395–404 (1998).
Hondowicz, B. D. et al. Discovery of T cell antigens by high-throughput screening of synthetic minigene libraries. PLoS One 7, e29949 (2012).
Wooldridge, L. et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J. Biol. Chem. 287, 1168–1177 (2012).
Wucherpfennig, K. W. et al. Polyspecificity of T cell and B cell receptor recognition. Semin. Immunol. 19, 216–224 (2007).
Holler, P. D. et al. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc. Natl Acad. Sci. USA 97, 5387–5392 (2000).
Frankiw, L., Baltimore, D. & Li, G. Alternative mRNA splicing in cancer immunotherapy. Nat. Rev. Immunol. 19, 675–687 (2019).
Jurtz, V. et al. NetMHCpan-4.0: improved peptide-MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J. Immunol. 199, 3360–3368 (2017).
Bassani-Sternberg, M. et al. Deciphering HLA-I motifs across HLA peptidomes improves neo-antigen predictions and identifies allostery regulating HLA specificity. PLOS Comput. Biol. 13, e1005725 (2017).
Kawakami, Y. et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl Acad. Sci. USA 91, 3515–3519 (1994). This study, along with that of van der Bruggen et al.21, constitutes pioneering T cell antigen-discovery work that identified several classic melanoma antigens.
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Sahin, U. et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl Acad. Sci. USA 92, 11810–11813 (1995).
Bethune, M. T. et al. Isolation and characterization of NY-ESO-1-specific T cell receptors restricted on various MHC molecules. Proc. Natl Acad. Sci. USA 115, E10702–E10711 (2018).
Robbins, P. F. et al. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 183, 1185–1192 (1996).
Wong, F. S. et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 5, 1026–1031 (1999).
McCutcheon, M. et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J. Immunol. Methods 210, 149–166 (1997).
Ogunshola, F. et al. Dual HLA B*42 and B*81-reactive T cell receptors recognize more diverse HIV-1 Gag escape variants. Nat. Commun. 9, 5023 (2018).
Koh, S. et al. A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol. Ther. Nucleic Acids 2, e114 (2013).
Joglekar, A. V. et al. T cell receptors for the HIV KK10 epitope from patients with differential immunologic control are functionally indistinguishable. Proc. Natl Acad. Sci. USA 115, 1877–1882 (2018).
Bertoletti, A. et al. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J. Exp. Med. 180, 933–943 (1994).
Mottez, E. et al. A single-chain murine class I major transplantation antigen. Eur. J. Immunol. 21, 467–471 (1991).
Uger, R. A., Barber, B. H. & Creating, C. T. L. targets with epitope-linked beta 2-microglobulin constructs. J. Immunol. 160, 1598–1605 (1998).
Yu, Y. Y., Netuschil, N., Lybarger, L., Connolly, J. M. & Hansen, T. H. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J. Immunol. 168, 3145–3149 (2002).
Kim, S. et al. Single-chain HLA-A2 MHC trimers that incorporate an immunodominant peptide elicit protective T cell immunity against lethal West Nile virus infection. J. Immunol. 184, 4423–4430 (2010).
Linnemann, C. et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85 (2015).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996). This study described the use of pMHC tetramers for identification and characterization of antigen-specific T lymphocytes.
Dolton, G. et al. More tricks with tetramers: a practical guide to staining T cells with peptide-MHC multimers. Immunology 146, 11–22 (2015).
Klenerman, P., Cerundolo, V. & Dunbar, P. R. Tracking T cells with tetramers: new tales from new tools. Nat. Rev. Immunol. 2, 263–272 (2002).
Wooldridge, L. et al. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology 126, 147–164 (2009).
Chen, H. et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat. Immunol. 13, 691–700 (2012).
Yang, J. D. et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog. 14, e1007060 (2018).
Toebes, M. et al. Design and use of conditional MHC class I ligands. Nat. Med. 12, 246–251 (2006).
Bakker, A. H. et al. Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, -A11, and -B7. Proc. Natl Acad. Sci. USA 105, 3825–3830 (2008).
Saini, S. K. et al. Dipeptides catalyze rapid peptide exchange on MHC class I molecules. Proc. Natl Acad. Sci. USA 112, 202–207 (2015).
Bethune, M. T., Comin-Anduix, B., Hwang Fu, Y. H., Ribas, A. & Baltimore, D. Preparation of peptide-MHC and T-cell receptor dextramers by biotinylated dextran doping. Biotechniques 62, 123–130 (2017).
Luimstra, J. J. et al. A flexible MHC class I multimer loading system for large-scale detection of antigen-specific T cells. J. Exp. Med. 215, 1493–1504 (2018).
Hadrup, S. R. et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat. Methods 6, 520–526 (2009).
Newell, E. W., Klein, L. O., Yu, W. & Davis, M. M. Simultaneous detection of many T-cell specificities using combinatorial tetramer staining. Nat. Methods 6, 497–499 (2009).
van Rooij, N. et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–e442 (2013).
Newell, E. W., Sigal, N., Bendall, S. C., Nolan, G. P. & Davis, M. M. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36, 142–152 (2012).
Ornatsky, O., Baranov, V. I., Bandura, D. R., Tanner, S. D. & Dick, J. Multiple cellular antigen detection by ICP-MS. J. Immunol. Methods 308, 68–76 (2006).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Newell, E. W. et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 31, 623–629 (2013).
Fehlings, M. et al. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat. Commun. 8, 562 (2017).
Rammensee, H. G., Falk, K. & Rötzschke, O. Peptides naturally presented by MHC class I molecules. Annu. Rev. Immunol. 11, 213–244 (1993).
Robins, H. S. et al. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood 114, 4099–4107 (2009).
Stevanović, S. & Schild, H. Quantitative aspects of T cell activation—peptide generation and editing by MHC class I molecules. Semin. Immunol. 11, 375–384 (1999).
Bentzen, A. K. et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 34, 1037–1045 (2016). This work, along with the study by Zhang et al.62, described DNA-barcode-based pMHC multimer technology to access peptide specificity and decipher TCR αβ TCR sequences in large scale.
Saini, S. K. et al. Empty peptide-receptive MHC class I molecules for efficient detection of antigen-specific T cells. Semin. Immunol. 4, eaau9039 (2019).
Bentzen, A. K. et al. T cell receptor fingerprinting enables in-depth characterization of the interactions governing recognition of peptide-MHC complexes. Nat. Biotechnol. 36, 1191–1196 (2018).
Pedersen, N. W. et al. CD8+ T cells from patients with narcolepsy and healthy controls recognize hypocretin neuron-specific antigens. Nat. Commun. 10, 837 (2019).
Zhang, S. Q. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat. Biotechnol. 36, 1156–1159 (2018).
Xu, Q., Schlabach, M. R., Hannon, G. J. & Elledge, S. J. Design of 240,000 orthogonal 25mer DNA barcode probes. Proc. Natl Acad. Sci. USA 106, 2289–2294 (2009).
Peng, S. et al. Sensitive detection and analysis of neoantigen-specific T cell populations from tumors and Blood. Cell Rep 28, 2728–2738 (2019).
Segaliny, A. I. et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 18, 3733–3749 (2018). This study utilized droplet microfluidics technology for functional screening and real-time monitoring of single TCR T cell activation upon recognition of target tumor cells.
Varadarajan, N. et al. Rapid, efficient functional characterization and recovery of HIV-specific human CD8+ T cells using microengraving. Proc. Natl Acad. Sci. USA 109, 3885–3890 (2012).
Ng, A. H. C. et al. MATE-Seq: microfluidic antigen-TCR engagement sequencing. Lab Chip 19, 3011–3021 (2019).
Hemmer, B. et al. Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J. Exp. Med. 185, 1651–1659 (1997).
Gavin, M. A., Dere, B., Grandea, A. G. III, Hogquist, K. A. & Bevan, M. J. Major histocompatibility complex class I allele-specific peptide libraries: identification of peptides that mimic an H-Y T cell epitope. Eur. J. Immunol. 24, 2124–2133 (1994).
Pinilla, C. et al. Combinatorial peptide libraries as an alternative approach to the identification of ligands for tumor-reactive cytolytic T lymphocytes. Cancer Res. 61, 5153–5160 (2001).
Gavin, M. A. & Bevan, M. J. Major histocompatibility complex allele-specific peptide libraries and identification of T-cell mimotopes. Methods Mol. Biol. 87, 235–248 (1998).
Wilson, D. B. et al. Immunogenicity. I. Use of peptide libraries to identify epitopes that activate clonotypic CD4+ T cells and induce T cell responses to native peptide ligands. J. Immunol. 163, 6424–6434 (1999).
Hiemstra, H. S. et al. The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc. Natl Acad. Sci. USA 94, 10313–10318 (1997).
Rubio-Godoy, V. et al. Combinatorial peptide library-based identification of peptide ligands for tumor-reactive cytolytic T lymphocytes of unknown specificity. Eur. J. Immunol. 32, 2292–2299 (2002).
Sherev, T., Wiesmüller, K. H. & Walden, P. Mimotopes of tumor-associated T-cell epitopes for cancer vaccines determined with combinatorial peptide libraries. Mol. Biotechnol. 25, 53–61 (2003).
Linnemann, T. et al. Mimotopes for tumor-specific T lymphocytes in human cancer determined with combinatorial peptide libraries. Eur. J. Immunol. 31, 156–165 (2001).
Nino-Vasquez, J. J. et al. A powerful combination: the use of positional scanning libraries and biometrical analysis to identify cross-reactive T cell epitopes. Mol. Immunol. 40, 1063–1074 (2004).
Barber, J. S. et al. Peptide library-based evaluation of T-cell receptor breadth detects defects in global and regulatory activation in human immunologic diseases. Proc. Natl Acad. Sci. USA 110, 8164–8169 (2013).
Ernst, W. et al. Baculovirus surface display: construction and screening of a eukaryotic epitope library. Nucleic Acids Res. 26, 1718–1723 (1998).
Kozono, H., White, J., Clements, J., Marrack, P. & Kappler, J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature 369, 151–154 (1994).
Szardenings, M. Phage display of random peptide libraries: applications, limits, and potential. J. Recept. Signal Transduct. Res. 23, 307–349 (2003).
Crawford, F., Huseby, E., White, J., Marrack, P. & Kappler, J. W. Mimotopes for alloreactive and conventional T cells in a peptide-MHC display library. PLoS Biol. 2, E90 (2004).
Wang, Y. et al. Using a baculovirus display library to identify MHC class I mimotopes. Proc. Natl Acad. Sci. USA 102, 2476–2481 (2005).
Crawford, F. et al. Use of baculovirus MHC/peptide display libraries to characterize T-cell receptor ligands. Immunol. Rev. 210, 156–170 (2006).
Wen, F., Sethi, D. K., Wucherpfennig, K. W. & Zhao, H. Cell surface display of functional human MHC class II proteins: yeast display versus insect cell display. Protein Eng. Des. Sel. 24, 701–709 (2011).
Birnbaum, M. E., Dong, S. & Garcia, K. C. Diversity-oriented approaches for interrogating T-cell receptor repertoire, ligand recognition, and function. Immunol. Rev. 250, 82–101 (2012).
Brophy, S. E., Holler, P. D. & Kranz, D. M. A yeast display system for engineering functional peptide-MHC complexes. J. Immunol. Methods 272, 235–246 (2003).
Boder, E. T. & Wittrup, K. D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 15, 553–557 (1997). This report describes yeast display of combinatorial polypeptide libraries.
Kieke, M. C., Cho, B. K., Boder, E. T., Kranz, D. M. & Wittrup, K. D. Isolation of anti-T cell receptor scFv mutants by yeast surface display. Protein Eng. 10, 1303–1310 (1997).
Boder, E. T., Bill, J. R., Nields, A. W., Marrack, P. C. & Kappler, J. W. Yeast surface display of a noncovalent MHC class II heterodimer complexed with antigenic peptide. Biotechnol. Bioeng. 92, 485–491 (2005).
Wen, F., Esteban, O. & Zhao, H. Rapid identification of CD4+ T-cell epitopes using yeast displaying pathogen-derived peptide library. J. Immunol. Methods 336, 37–44 (2008).
Wen, F. & Zhao, H. Construction and screening of an antigen-derived peptide library displayed on yeast cell surface for CD4+ T cell epitope identification. Methods Mol. Biol. 1061, 245–264 (2013).
Adams, J. J. et al. T cell receptor signaling is limited by docking geometry to peptide-major histocompatibility complex. Immunity 35, 681–693 (2011).
Gee, M. H. et al. Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. Cell 172, 549–563 (2018).
Birnbaum, M. E. et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014).
Starwalt, S. E., Masteller, E. L., Bluestone, J. A. & Kranz, D. M. Directed evolution of a single-chain class II MHC product by yeast display. Protein Eng. 16, 147–156 (2003).
Davis, M. M. & Boyd, S. D. Recent progress in the analysis of αβT cell and B cell receptor repertoires. Curr. Opin. Immunol. 59, 109–114 (2019).
Joglekar, A. V. et al. T cell antigen discovery via signaling and antigen-presenting bifunctional receptors. Nat. Methods 16, 191–198 (2019). This study, along with Kisielow et al.99, Kula et al.100, Li et al.101 and Sharma et al.102, described cell-based epitope discovery methods.
Kisielow, J., Obermair, F.-J. & Kopf, M. Deciphering CD4+ T cell specificity using novel MHC-TCR chimeric receptors. Nat. Immunol. 20, 652–662 (2019).
Kula, T. et al. T-Scan: a genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028 (2019).
Li, G. et al. T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019).
Sharma, G., Rive, C. M. & Holt, R. A. Rapid selection and identification of functional CD8+ T cell epitopes from large peptide-coding libraries. Nat. Commun. 10, 4553 (2019).
Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 4, 815 (2003).
Emerson, R. O. et al. Immunosequencing identifies signatures of cytomegalovirus exposure history and HLA-mediated effects on the T cell repertoire. Nat. Genet. 49, 659–665 (2017).
DeWitt, W. S. III et al. Human T cell receptor occurrence patterns encode immune history, genetic background, and receptor specificity. Elife 7, e38358 (2018).
Huth, A., Liang, X., Krebs, S., Blum, H. & Moosmann, A. Antigen-specific TCR signatures of cytomegalovirus infection. J. Immunol. 202, 979–990 (2019).
Dash, P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017). This study, along with Glanville et al.108, reported major advances in predicting epitope specificity based on TCR sequence.
Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547, 94–98 (2017).
Lanzarotti, E., Marcatili, P. & Nielsen, M. T-cell receptor cognate target prediction based on paired α and β chain sequence and structural CDR loop similarities. Front. Immunol. 10, 2080 (2019).
Ostmeyer, J., Christley, S., Toby, I. T. & Cowell, L. G. Biophysicochemical motifs in T-cell receptor sequences distinguish repertoires from tumor-infiltrating lymphocyte and adjacent healthy tissue. Cancer Res. 79, 1671–1680 (2019).
Li, B. et al. Investigation of antigen-specific T cell receptor clusters in human cancers. Clin. Cancer Res. 26, 1359–1371 (2019).
Carter, J. A. et al. Single T cell sequencing demonstrates the functional role of αβ TCR pairing in cell lineage and antigen specificity. Front. Immunol. 10, 1516 (2019).
Singh, N. K. et al. Emerging concepts in TCR specificity: rationalizing and (maybe) predicting outcomes. J. Immunol. 199, 2203–2213 (2017).
Acknowledgements
We thank K. Ford and K. Rankin for comments and suggestions on the manuscript. The figures were created with BioRender.com. This work was supported by the National Natural Science Foundation (81972875), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2019PT310028) and The CAMS Initiative for Innovative Medicine (2016-I2M-1-005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nicole Rusk and Lin Tang were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joglekar, A.V., Li, G. T cell antigen discovery. Nat Methods 18, 873–880 (2021). https://doi.org/10.1038/s41592-020-0867-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-020-0867-z
This article is cited by
-
A biomimetic robotic antigen-presenting system for sensitive T cell recognition
Nature Methods (2024)
-
Single-cell immune repertoire analysis
Nature Methods (2024)
-
Multimodal probing of T-cell recognition with hexapod heterostructures
Nature Methods (2024)
-
Integrating single-cell multi-omics and prior biological knowledge for a functional characterization of the immune system
Nature Immunology (2024)
-
A robust deep learning workflow to predict CD8 + T-cell epitopes
Genome Medicine (2023)