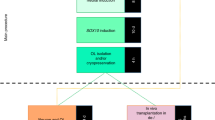
Abstract
Cerebral organoids provide an accessible system for investigations of cellular composition, interactions, and organization but have lacked oligodendrocytes, the myelinating glia of the central nervous system. Here we reproducibly generated oligodendrocytes and myelin in ‘oligocortical spheroids’ derived from human pluripotent stem cells. Molecular features consistent with those of maturing oligodendrocytes and early myelin appeared by week 20 in culture, with further maturation and myelin compaction evident by week 30. Promyelinating drugs enhanced the rate and extent of oligodendrocyte generation and myelination, and spheroids generated from human subjects with a genetic myelin disorder recapitulated human disease phenotypes. Oligocortical spheroids provide a versatile platform for studies of myelination of the developing central nervous system and offer new opportunities for disease modeling and therapeutic development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284–20289 (2013).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 112, 15672–15677 (2015).
Jo, J. et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257 (2016).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017).
Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017).
Li, Y. et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 20, 385–396 (2017).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Renner, M. et al. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 36, 1316–1329 (2017).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 (2017).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398 (2017).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Pașca, S. P. The rise of three-dimensional human brain cultures. Nature 553, 437–445 (2018).
Arlotta, P. Organoids required! A new path to understanding human brain development and disease. Nat. Methods 15, 27–29 (2018).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Luo, C. et al. Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 17, 3369–3384 (2016).
Monzel, A. S. et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 8, 1144–1154 (2017).
McMorris, F. A., Smith, T. M., DeSalvo, S. & Furlanetto, R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA 83, 822–826 (1986).
Noble, M., Murray, K., Stroobant, P., Waterfield, M. D. & Riddle, P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333, 560–562 (1988).
Barres, B. A., Lazar, M. A. & Raff, M. C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108 (1994).
Jakovcevski, I., Filipovic, R., Mo, Z., Rakic, S. & Zecevic, N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front. Neuroanat. 3, 5 (2009).
Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268 (2016).
Bujalka, H. et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 11, e1001625 (2013).
James, D., Noggle, S. A., Swigut, T. & Brivanlou, A. H. Contribution of human embryonic stem cells to mouse blastocysts. Dev. Biol. 295, 90–102 (2006).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Nevin, Z. S. et al. Modeling the mutational and phenotypic landscapes of Pelizaeus-Merzbacher disease with human iPSC-derived oligodendrocytes. Am. J. Hum. Genet. 100, 617–634 (2017).
Weidenheim, K. M., Kress, Y., Epshteyn, I., Rashbaum, W. K. & Lyman, W. D. Early myelination in the human fetal lumbosacral spinal cord: characterization by light and electron microscopy. J. Neuropathol. Exp. Neurol. 51, 142–149 (1992).
Szuchet, S., Nielsen, L. L., Domowicz, M. S., Austin, J. R. II & Arvanitis, D. L. CNS myelin sheath is stochastically built by homotypic fusion of myelin membranes within the bounds of an oligodendrocyte process. J. Struct. Biol. 190, 56–72 (2015).
Wang, S. et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264 (2013).
Windrem, M. S. et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21, 195–208 (2017).
Gao, F. B., Durand, B. & Raff, M. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr. Biol. 7, 152–155 (1997).
Raff, M. C., Lillien, L. E., Richardson, W. D., Burne, J. F. & Noble, M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333, 562–565 (1988).
Temple, S. & Raff, M. C. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell 44, 773–779 (1986).
Nowakowski, T. J. et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358, 1318–1323 (2017).
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Cohen, J. A. & Tesar, P. J. Clemastine fumarate for promotion of optic nerve remyelination. Lancet 390, 2421–2422 (2017).
Green, A. J. et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017).
Hobson, G. M. & Garbern, J. Y. Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders. Semin. Neurol. 32, 62–67 (2012).
Douvaras, P. et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 3, 250–259 (2014).
Axten, J. M. et al. Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med. Chem. Lett. 4, 964–968 (2013).
Garbern, J. Y. Pelizaeus-Merzbacher disease: genetic and cellular pathogenesis. Cell. Mol. Life Sci. 64, 50–65 (2007).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449 (2017).
Mariani, J. et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 (2015).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Pamies, D. et al. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34, 362–376 (2017).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179 (2006).
Miller, D. J. et al. Prolonged myelination in human neocortical evolution. Proc. Natl Acad. Sci. USA 109, 16480–16485 (2012).
Sheng, Y. et al. Using iPSC-derived human DA neurons from opioid-dependent subjects to study dopamine dynamics. Brain Behav. 6, e00491 (2016).
Najm, F. J. et al. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nat. Methods 8, 957–962 (2011).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Acknowledgements
This research was supported by the NIH (R01NS093357 to P.J.T.; R01NS095280 to P.J.T. and R.H.M.; T32GM007250 and F30HD084167 to Z.S.N.), the Pelizaeus-Merzbacher Disease Foundation (P.J.T.), the New York Stem Cell Foundation (P.J.T.), the Connor B. Judge Foundation (P.J.T.), the New York Stem Cell Foundation Research Institute (V.F.), the National Stem Cell Foundation (T.J. and V.F.), and philanthropic support from the Peterson, Fakhouri, Long, Goodman, Geller, Galbut/Heil, and Weidenthal families. Additional support was provided by the CWRU SOM Light Microscopy Core Facility (S10-OD016164) and the Genomics core facility of the Case Comprehensive Cancer Center (P30CA043703). We are grateful to B. Nawash, C. Blake, M. Cartwright, M. Cameron, R. Lee, A. Miron, S. Edelheit, M. Hitomi, F. Pirozzi, and A. Wynshaw-Boris for technical assistance and discussion. Rat anti-PLP1 was a gift from W. Macklin (University of Colorado Anschutz Medical Campus, Aurora, CO, USA); rabbit anti-MYRF was provided by M. Wegner (Institut für Biochemie, Emil-Fischer-Zentrum, Universität Erlangen-Nürnberg, Erlangen, Germany).
Author information
Authors and Affiliations
Contributions
M.M., Z.S.N., and P.J.T. conceived and initiated the project. M.M. and Z.S.N. developed the oligocortical spheroid protocol and generated spheroids for all experiments. M.M., H.E.S., B.L.L.C., K.C.A., and L.B. performed immunohistochemistry and quantification and generated associated figures. D.C.F. and B.L.L.C. analyzed RNA-seq data and generated associated figures. E.G., C.C.-P., M.K., and R.H.M. designed and performed electron microscopy experiments and analysis and generated associated figures. H.E.S. maintained hPSC lines. T.J., P.D., and V.F. independently replicated the oligocortical spheroid protocol. Z.S.N., M.M., and P.J.T. wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
P.J.T. and R.H.M. are consultants for Convelo Therapeutics, which has licensed patents from Case Western Reserve University. P.J.T., R.H.M., and Case Western Reserve University hold equity in Convelo Therapeutics. D.C.F. became an employee of Convelo Therapeutics subsequent to the completion of these studies. P.J.T. is a consultant and on the Scientific Advisory Board of Cell Line Genetics. P.J.T. is chair of the Scientific Advisory Board (volunteer position) for the Pelizaeus-Merzbacher Disease Foundation.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Generation of oligodendrocyte precursor cells in human cortical spheroids.
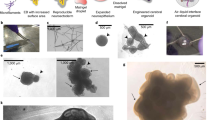
a, Schematic of spheroid generation. The protocols to generate neurocortical spheroids (NCS) and oligocortical spheroids (OCS) were the same until week 8. Neurocortical spheroids were grown in basal media, while oligocortical spheroids were treated with PDGF-AA/IGF-1 to generate OPCs from day 50-60. Increase in OPC numbers was assessed at the end of week 9. Colors in the schematic simulate neurons (magenta), astrocytes (red) and OPCs/Oligodendrocytes (green). b-c, Representative fluorescence images of week 8 (b) and week 9 (c) H7 spheroids generated with the neurocortical protocol. These spheroids do not generate OPCs (OLIG2:yellow and SOX10:mageneta). Scale bar, 50μm for b-d. d, Representative fluorescence image of week 9, H7 spheroids generated with the oligocortical protocol up through treatment with PDGF-AA/IGF-1. These spheroids generate OPCs (OLIG2:yellow and SOX10:mageneta). Arrows show OLIG2/SOX10 double-positive cells. e, Quantification of OLIG2-positive and SOX10/OLIG2-double positive OPCs in week 9 spheroids generated with the neurocortical or oligocortical protocol. Cells were counted from three planes each from five individual spheroids (colored points) of lines H7, H9 and CWRU191 and averaged (white boxes). Error bars are standard deviation, n=5 spheroids from the same batch per line.
Supplementary Figure 2 Validation of the oligocortical protocol in three additional human pluripotent cell lines.
a, Representative fluorescence images of PLP1 in week 14 oligocortical spheroids generated from H9, CWRU191, and RUES1. Similar results were obtained from 3 independent batches of spheroids for H9, CWRU191 and CWRU198 and one batch of RUES1. Scale bar, 50μm. b, Representative fluorescence images of MYRF in week 14 oligocortical spheroids generated from H9, CWRU191, and RUES1 Similar results were obtained from 3 independent batches of spheroids for H9, CWRU191 and CWRU198 and one batch of RUES1. Scale bar, 50μm. c, Schematic of MYRF quantification in Figure 1d with representative fluorescence images of MYRF in a single week 14 oligocortical spheroid generated from H7. The four panels (1-4) demonstrate four equally magnified, equally sized, and consistently distributed areas that were imaged and counted per spheroid. The reported %MYRF-positive cells per spheroid is the average of these four images. Scale bar, 50μm.
Supplementary Figure 3 Maturation of oligodendrocytes from additional pluripotent cell lines.
a, Representative fluorescence images of MYRF and PLP1 expression in week 20, H9, CWRU191, and RUES1 oligocortical spheroids. Results are representative of spheroids generated from 2 independent batches of lines H9 and CWRU191 and 1 batch of line RUES1. Scale bar, 50μm. b, Representative EM images of multiple loosely compacted myelin wraps around axons in week 20, H9 and CWRU191 oligocortical spheroids. EM analysis was performed on 3 spheroids from the same batch for each line. EM analysis of RUES1 was not performed. Scale bar, 1μm. c, Representative fluorescence images of Sox10 and MYRF expression in week 14 and 20 H7 oligocortical spheroids. Results are representative of spheroids generated from 2 independent batches. Scale bar, 50μm.
Supplementary Figure 4 BrdU-based fate mapping of oligodendrocytes in oligocortical spheroids.
a, Representative fluorescence images of two additional H7, and two H9, and two CWRU191 spheroids generated with the oligocortical protocol up through PDGF-AA/IGF-1 treatment, then administered two doses of BrdU during week 9 (day 58 and 60) to label dividing cells. After the second BrdU pulse, a majority of BrdU-positive (magenta) cells localize with SOX2-positive (yellow) and Vimentin-positive (blue) cells. By Week 14, some of the BrdU labelled cells are double-positive (arrows in high magnification inset) for the oligodendrocyte marker MYRF (cyan). Pulse chase experiments were performed on a single batch of spheroids from each line, and 4 spheroids per line were analyzed. Scale bar, 50μm.
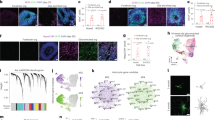
Supplementary Figure 5 Single-cell analysis of cell populations in week 12 oligocortical spheroids.
a, Clustering of single cell RNA-seq data from Week 12 H7 oligocortical spheroids compared to single cell human fetal brain cells generated by Nowakowski et al. 2017. A continuum of progenitor populations is evident in both data sets through visualization of progenitor markers Vimentin, SOX2, Nestin, and Sox6 while only the oligocortical spheroids show evidence of an emerging oligodendrocyte cluster (PLP1/DM20 and OMG). Single Cell RNA-seq was performed 10 spheroids from a single batch.
Supplementary Figure 6 CRISPR correction of a PLP1 point mutation.
a, Schematic of the correction of a PLP1 point mutation (PLP1c.254T>G) in patient-derived hiPSCs using a guide RNA overlapping the mutation and single strand antisense oligonucleotide donor. b, Sanger sequencing trace and karyotype of the mutant parental (PLP1c.254G) line. c, Sanger sequencing trace and karyotype of the corrected (PLP1c.254T) line.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6
Rights and permissions
About this article
Cite this article
Madhavan, M., Nevin, Z.S., Shick, H.E. et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods 15, 700–706 (2018). https://doi.org/10.1038/s41592-018-0081-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-018-0081-4
This article is cited by
-
Lessons from inducible pluripotent stem cell models on neuronal senescence in aging and neurodegeneration
Nature Aging (2024)
-
Gliomas: a reflection of temporal gliogenic principles
Communications Biology (2024)
-
Genetics of human brain development
Nature Reviews Genetics (2024)
-
Pervasive environmental chemicals impair oligodendrocyte development
Nature Neuroscience (2024)
-
A beginner’s guide on the use of brain organoids for neuroscientists: a systematic review
Stem Cell Research & Therapy (2023)