Abstract
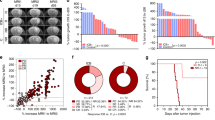
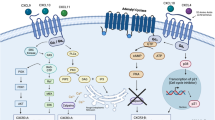
Glioblastoma is the most common primary central nervous system malignancy and has a poor prognosis. Standard first-line treatment, which includes surgery followed by adjuvant radio-chemotherapy, produces only modest benefits to survival1,2. Here, to explore the feasibility, safety and immunobiological effects of PD-1 blockade in patients undergoing surgery for glioblastoma, we conducted a single-arm phase II clinical trial (NCT02550249) in which we tested a presurgical dose of nivolumab followed by postsurgical nivolumab until disease progression or unacceptable toxicity in 30 patients (27 salvage surgeries for recurrent cases and 3 cases of primary surgery for newly diagnosed patients). Availability of tumor tissue pre- and post-nivolumab dosing and from additional patients who did not receive nivolumab allowed the evaluation of changes in the tumor immune microenvironment using multiple molecular and cellular analyses. Neoadjuvant nivolumab resulted in enhanced expression of chemokine transcripts, higher immune cell infiltration and augmented TCR clonal diversity among tumor-infiltrating T lymphocytes, supporting a local immunomodulatory effect of treatment. Although no obvious clinical benefit was substantiated following salvage surgery, two of the three patients treated with nivolumab before and after primary surgery remain alive 33 and 28 months later.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The molecular data analyzed in the current study linked to patient anonymized IDs are available from corresponding author on reasonable request. Nanostring raw data are available as Supplementary Table 7.
References
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
Senft, C. et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 12, 997–1003 (2011).
Wick, W., Osswald, M., Wick, A. & Winkler, F. Treatment of glioblastoma in adults. Ther. Adv. Neurol. Disord. 11, 1–13 (2018).
Wick, W. et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 377, 1954–1963 (2017).
Chinot, O. L. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722 (2014).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378, 1789–1801 (2018).
Wainwright, D. A. et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 20, 5290–5301 (2014).
Ashizawa, T. et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin. Cancer Res. 23, 149–158 (2017).
Kim, J. E. et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 23, 124–136 (2017).
Nduom, E. K. et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-oncol. 18, 195–205 (2016).
Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018).
Reardon, D. A. et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-oncol. 19, iii21 (2017).
Reiss, S. N., Yerram, P., Modelevsky, L. & Grommes, C. Retrospective review of safety and efficacy of programmed cell death-1 inhibitors in refractory high grade gliomas. J. Immunother. Cancer 5, 99 (2017).
Melero, I., Berraondo, P., Rodriguez-Ruiz, M. E. & Perez-Gracia, J. L. Making the most of cancer surgery with neoadjuvant immunotherapy. Cancer Discov. 6, 1312–1314 (2016).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Wick, W. et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 10, 372–385 (2014).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Korn, T. & Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 17, 179–194 (2017).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. (in the press).
Melero, I. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472 (2015).
Reardon, D. A. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4, 124–135 (2016).
Liau, L. M. et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 16, 142 (2018).
Antonios, J. P. et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight 1, e87059 (2016).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
John, L. B. et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 19, 5636–5646 (2013).
Liu, Y. et al. Targeting myeloid-derived suppressor cells for cancer immunotherapy. Cancer Immunol. Immunother. 67, 1181–1195 (2018).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Lang, F. F. et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 36, 1419–1427 (2018).
Johnson, W. E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 15, 550 (2014).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Shannon, C. E. The mathematical theory of communication. 1963. MD Comput. 14, 306–317 (1997).
Acknowledgements
We thank R. Latek and D. McDonald from Bristol-Myers Squibb and the study coordinators and staff from our Clinical Research Unit, E. Guirado, L. Resano and B. Palencia for project coordination. We also thank Laura Johnson and Josh Haimes from ArcherDx for experimental and technical support. Financial support was through the Immunoncology Network (I-ON) supported by Bristol-Myers Squibb. Additional funds came to K.A.S. from Department of Defense LCRP Career Development Award W81XWH-16-1-0160, NIH Lung SPORE in Lung Cancer P50CA196530, and Stand Up To Cancer Translational Research Grants SU2C-AACR-DT17-15 and SU2C-AACR-DT22-17, and to I.M. from MINECO (SAF2014-52361-R and 2017-83267-C2-1-R), Fundación de la Asociación Española Contra el Cáncer (AECC), Fundación BBVA. M.E.R.-R. received a Rio Hortega contract from ISCIII.
Author information
Authors and Affiliations
Contributions
K.A.S. designed research, analyzed data and wrote the draft of the manuscript and revised the manuscript. M.E.R.-R. wrote the study protocol, treated patients, analyzed data, prepared figures and revised the manuscript. R.D.-V. performed neurosurgery operations, evaluated clinical results and revised the manuscript. A.L.-J. performed experiments, analyzed data, prepared figures and revised the manuscript. A.P. performed experiments, analyzed data and prepared figures. M.A.I. collected and processed histology samples. S.I. performed flow cytometry experiments and prepared figures. C.d.A. collected and processed histology samples and analyzed data. A.L.-D.d.C. performed flow cytometry experiments. S.T. performed neurosurgery operations, evaluated clinical results and revised the manuscript. P.B. analyzed data, prepared figures and revised the manuscript. F.V.-E. performed experiments and revised the manuscript. J.C. performed statistical analyses and revised the manuscript. A.G. treated patients and analyzed data. M.G. was the clinical pharmacist in charge of the clinical trial. I.G. was the study coordinator and staff nurse in the clinical trial. J.G.P.-L. treated patients and analyzed data. M.F.S. analyzed data and revised the manuscript. J.L.P.-G. wrote the study protocol, obtained financial support, treated patients, analyzed data, prepared figures and drafted the manuscript. I.M. designed the study, obtained financial support, supervised patient treatment, evaluated and analyzed data and drafted the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.A.S. is a consultant for Celgene, Moderna Therapeutics and Shattuck Labs and has received research funding from Vasculox, Moderna, Takeda, Surface Oncology, Tesaro, Pierre-Fabre, Merck and Bristol-Myers Squibb. I.M. is a consultant for BMS, Merck-Serono, Roche-Genetech, Tusk, Alligator, Genmab, Molecular Partners, F-Star, Bayer, Seattle Genetics and Alligator and has received research funding from BMS, Roche, Bioncotech and Alligator. J.L.P.-G. is a consultant for and has received research support, travel grants and lecture fees from BMS and Roche as well as research support and travel grants from MSD.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Swimmer plot graph of treated patients.
Progression of patients included in the trial in months, including subsequent anticancer treatments that the patients received. Duration of nivolumab treatment is also included. DT, death from tumor; DO, death from other cause; RT, radiotherapy; TMZ, temozolamide; UK, unknown.
Extended Data Fig. 2 PD-1 receptor occupancy on tumor-infiltrating CD8 T cells.
Cell suspensions, as in Fig. 2f, from three indicated cases that had sufficient CD8 cells with PD-1 surface expression were immunostained with a nivolumab-competing monoclonal antibody and with a non-competing anti-PD-1 monoclonal antibody. Top, mean intensity of fluorescence (MFI) of cells. Bottom, percentage of positive cells.
Extended Data Fig. 3 TCRγδ clonality assessments by mRNA sequencing of tumor samples before and after surgical resection.
a,b, Charts showing the number of TRG (a) and TRD clones (b) detected in pre- and postsurgical samples of patients from the neoadjuvant nivolumab and control groups. Only cases with detectable TRD sequences are shown. P values were obtained using paired two-tailed Wilcoxon rank-sum tests and corrected with Benjamini–Hochberg method and are indicated above each graph. ns, not significant (P < 0.05).
Supplementary information
Supplementary information
Supplementary Tables 1–6.
Supplementary Table 7
Supplementary Table 7
Rights and permissions
About this article
Cite this article
Schalper, K.A., Rodriguez-Ruiz, M.E., Diez-Valle, R. et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 25, 470–476 (2019). https://doi.org/10.1038/s41591-018-0339-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0339-5
This article is cited by
-
Clinical activity and safety of sintilimab, bevacizumab, and TMZ in patients with recurrent glioblastoma
BMC Cancer (2024)
-
Novel Clinical Trials and Approaches in the Management of Glioblastoma
Current Oncology Reports (2024)
-
Current approaches in glioblastoma multiforme immunotherapy
Clinical and Translational Oncology (2024)
-
Global research trends in tongue cancer from 2000 to 2022: bibliometric and visualized analysis
Clinical Oral Investigations (2024)
-
Glioblastom – aktuelle Therapiekonzepte
Die Onkologie (2024)