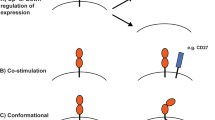
Abstract
γδ T cells have been retained as a lineage over the majority of vertebrate evolution, are able to respond to immune challenges in unique ways, and are of increasing therapeutic interest. However, one central mystery has endured: the identity of the ligands recognized by the γδ T cell antigen receptor. Here we discuss the inherent challenges in answering this question, the new opportunities provided by recent studies, and the criteria by which the field might judge success.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 February 2019
In the version of this article initially published, the affiliations were incorrect. The correct affiliations are as follows: “1Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK. 2Institute of Immunology and Immunotherapy, Cancer Immunology and Immunotherapy Centre, Cancer Research UK Birmingham Centre, University of Birmingham, Birmingham, UK.” The reference citation at the end of the first sentence of the second paragraph of the subsection ‘A perspective on current methods of ligand identification’ was incorrect; the correct citation is “...ligands20–40.” There is an error (en dash) in the fourth paragraph of that section; the correct text is “...specific for CD1 and phycoerythrin...”. There is an error (“proposed”) in the fourth paragraph of the subsection ‘An emerging adaptive perspective on antigenic γδ TCR ligands’; the correct text is “...are suggested to recognize...”. There is an error (“via”) in the fourth paragraph of the subsection ‘B7-like molecules as candidate γδ TCR ligands’; the correct text is “...in a non-clonotypic fashion are striking...”. Finally, reference citations throughout the legend to Fig. 1 are incorrect; the correct citations are as follows: MHC class I free heavy chain22; HLA-B580234; I-Ek (ref. 30); MSH2 (MutShomolog 2) and HSP60 (heat-shock protein 60)24; ULBP4 (UL16-binding protein 4)27; MICA41; the herpes simplex virus glycoprotein HSVgI33; ATPase–apolipoprotein-AI39; and insulin B:9-23 peptide antigen40. The errors have been corrected in the HTML and PDF versions of the article.
References
Hayday, A. C. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 (2000).
Hirano, M. et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 501, 435–438 (2013).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
De Libero, G. et al. Selection by two powerful antigens may account for the presence of the major population of human peripheral γδ T cells. J. Exp. Med. 173, 1311–1322 (1991).
Khairallah, C., Déchanet-Merville, J. & Capone, M. γδ T cell-mediated immunity to cytomegalovirus infection. Front. Immunol. 8, 105 (2017).
Khairallah, C. et al. γδ T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog. 11, e1004702 (2015).
Sell, S. et al. Control of murine cytomegalovirus infection by γδ T cells. PLoS Pathog. 11, e1004481 (2015).
Groh, V., Steinle, A., Bauer, S. & Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 (1998).
Halary, F. et al. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
García, V. E. et al. IL-15 enhances the response of human γδ T cells to nonpeptide microbial antigens. J. Immunol. 160, 4322–4329 (1998).
García, V. E. et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162, 6114–6121 (1999).
Rincon-Orozco, B. et al. Activation of Vγ9Vδ2 T cells by NKG2D. J. Immunol. 175, 2144–2151 (2005).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 (2008).
Kalyan, S. & Kabelitz, D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell. Mol. Immunol. 10, 21–29 (2013).
Grusby, M. J. et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl Acad. Sci. USA 90, 3913–3917 (1993).
Davis, M. M. & Bjorkman, P. J. T-cell antigen receptor genes and T-cell recognition. Nature 334, 395–402 (1988).
Gomes, A. Q., Martins, D. S. & Silva-Santos, B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 70, 10024–10027 (2010).
Bai, L. et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur. J. Immunol. 42, 2505–2510 (2012).
Barbee, S. D. et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc. Natl Acad. Sci. USA 108, 3330–3335 (2011).
Bonneville, M. et al. Chicago 2014-30 years of γδ T cells. Cell. Immunol. 296, 3–9 (2015).
Bruder, J. et al. Target specificity of an autoreactive pathogenic human γδ-T cell receptor in myositis. J. Biol. Chem. 287, 20986–20995 (2012).
Chen, H. et al. Identification of human T cell receptor γδ-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J. Biol. Chem. 283, 12528–12537 (2008).
Crowley, M. P. et al. A population of murine γδ T cells that recognize an inducible MHC class Ib molecule. Science 287, 314–316 (2000).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Kong, Y. et al. The NKG2D ligand ULBP4 binds to TCRγ9/δ2 and induces cytotoxicity to tumor cells through both TCRγδ and NKG2D. Blood 114, 310–317 (2009).
Luoma, A. M. et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 39, 1032–1042 (2013).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA 114, 3163–3168 (2017).
Matis, L. A. et al. Structure and specificity of a class II MHC alloreactive γδ T cell receptor heterodimer. Science 245, 746–749 (1989).
Melandri, D. et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19, 1352–1365 (2018).
Roy, S. et al. Molecular analysis of lipid-reactive Vδ1 γδ T cells identified by CD1c tetramers. J. Immunol. 196, 1933–1942 (2016).
Sciammas, R. & Bluestone, J. A. HSV-1 glycoprotein I-reactive TCR γδ cells directly recognize the peptide backbone in a conformationally dependent manner. J. Immunol. 161, 5187–5192 (1998).
Silva-Santos, B., Schamel, W. W., Fisch, P. & Eberl, M. γδ T-cell conference 2012: close encounters for the fifth time. Eur. J. Immunol. 42, 3101–3105 (2012).
Uldrich, A. P. et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 14, 1137–1145 (2013).
Willcox, C. R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012).
Xu, B. et al. Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl Acad. Sci. USA 108, 2414–2419 (2011).
Zeng, X. et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 37, 524–534 (2012).
Scotet, E. et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22, 71–80 (2005).
Aydintug, M. K. et al. γδ T cells recognize the insulin B:9-23 peptide antigen when it is dimerized through thiol oxidation. Mol. Immunol. 60, 116–128 (2014).
Wu, J., Groh, V. & Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J. Immunol. 169, 1236–1240 (2002).
Exley, M. A. & Koziel, M. J. To be or not to be NKT: natural killer T cells in the liver. Hepatology 40, 1033–1040 (2004).
Rhodes, D. A., Reith, W. & Trowsdale, J. Regulation of immunity by butyrophilins. Annu. Rev. Immunol. 34, 151–172 (2016).
Vantourout, P. et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl Acad. Sci. USA 115, 1039–1044 (2018).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40, 490–500 (2014).
Vavassori, S. et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 14, 908–916 (2013).
Jönsson, P. et al. Remarkably low affinity of CD4/peptide-major histocompatibility complex class II protein interactions. Proc. Natl Acad. Sci. USA 113, 5682–5687 (2016).
Janeway, C. A. Jr., Jones, B. & Hayday, A. Specificity and function of T cells bearing γδ receptors. Immunol. Today 9, 73–76 (1988).
Di Marco Barros, R. et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell 167, 203–218 (2016).
Asarnow, D. M. et al. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell 55, 837–847 (1988).
Willcox, C. R., Davey, M. S. & Willcox, B. E. Development and selection of the human Vγ9Vδ2+ T-cell repertoire. Front. Immunol. 9, 1501 (2018).
Davey, M. S., Willcox, C. R., Baker, A. T., Hunter, S. & Willcox, B. E. Recasting human Vδ1 lymphocytes in an adaptive role. Trends Immunol. 39, 446–459 (2018).
Davey, M. S. et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9- subsets. Nat. Commun. 9, 1760 (2018).
Davey, M. S. et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017).
Davey, M. S., Willcox, C. R., Hunter, S., Oo, Y. H. & Willcox, B. E. V. Vδ2+ T cells—two subsets for the price of one. Front. Immunol. 9, 2106 (2018).
Hunter, S. et al. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 69, 654–665 (2018).
Ravens, S. et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017).
Hayday, A. C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Mamedov, M. R. et al. A macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363 (2018).
Burnet, F. M. A modification of Jerne’s theory of antibody production using the concept of clonal selection. Aust. J. Sci. 20, 67–69 (1957).
Lafarge, X. et al. Expression of MHC class I receptors confers functional intraclonal heterogeneity to a reactive expansion of γδ T cells. Eur. J. Immunol. 35, 1896–1905 (2005).
Ribot, J. C., Ribeiro, S. T., Correia, D. V., Sousa, A. E. & Silva-Santos, B. Human γδ thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 192, 2237–2243 (2014).
Hohlfeld, R., Engel, A. G., Ii, K. & Harper, M. C. Polymyositis mediated by T lymphocytes that express the γδ receptor. N. Engl. J. Med. 324, 877–881 (1991).
Pluschke, G., Rüegg, D., Hohlfeld, R. & Engel, A. G. Autoaggressive myocytotoxic T lymphocytes expressing an unusual γδ T cell receptor. J. Exp. Med. 176, 1785–1789 (1992).
Ugur, M., Kaminski, A. & Pabst, O. Lymph node γδ and αβ CD8+ T cells share migratory properties. Sci. Rep. 8, 8986 (2018).
Boyden, L. M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40, 656–662 (2008).
Chodaczek, G., Papanna, V., Zal, M. A. & Zal, T. Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 13, 272–282 (2012).
Salim, M. et al. Characterization of a putative receptor binding surface on Skint-1, a critical determinant of dendritic epidermal T cell selection. J. Biol. Chem. 291, 9310–9321 (2016).
Wang, H. & Morita, C. T. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 195, 4583–4594 (2015).
Gu, S., Borowska, M. T., Boughter, C. T. & Adams, E. J. Butyrophilin3A proteins and Vγ9Vδ2 T cell activation. Semin. Cell Dev. Biol. 84, 65–74 (2018).
Riaño, F. et al. Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur. J. Immunol. 44, 2571–2576 (2014).
Palakodeti, A. et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 287, 32780–32790 (2012).
Wang, H., Fang, Z. & Morita, C. T. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J. Immunol. 184, 6209–6222 (2010).
Lebrero-Fernández, C. et al. Altered expression of Butyrophilin (BTN) and BTN-like (BTNL) genes in intestinal inflammation and colon cancer. Immun. Inflamm. Dis. 4, 191–200 (2016).
Acknowledgements
Funded by a Wellcome Trust Investigator Award to B.E.W., supporting C.R.W. (099266/Z/12/Z). We would like to thank Professor Adrian Hayday, Dr Martin Davey, and Dr Pierre Vantourout for fruitful discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Willcox, B.E., Willcox, C.R. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol 20, 121–128 (2019). https://doi.org/10.1038/s41590-018-0304-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0304-y
This article is cited by
-
Adaptive immune receptor repertoire analysis
Nature Reviews Methods Primers (2024)
-
Local γδ T cells: translating promise to practice in cancer immunotherapy
British Journal of Cancer (2023)
-
Modulation of lytic molecules restrain serial killing in γδ T lymphocytes
Nature Communications (2023)
-
Double-edged sword: γδ T cells in mucosal homeostasis and disease
Experimental & Molecular Medicine (2023)
-
Innate lymphoid cells and innate-like T cells in cancer — at the crossroads of innate and adaptive immunity
Nature Reviews Cancer (2023)