Abstract
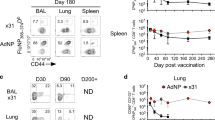
Memory B cells are found in lymphoid and non-lymphoid tissues, suggesting that some may be tissue-resident cells. Here we show that pulmonary influenza infection elicited lung-resident memory B cells (BRM cells) that were phenotypically and functionally distinct from their systemic counterparts. BRM cells were established in the lung early after infection, in part because their placement required local antigen encounter. Lung BRM cells, but not systemic memory B cells, contributed to early plasmablast responses following challenge infection. Following secondary infection, antigen-specific BRM cells differentiated in situ, whereas antigen-non-specific BRM cells were maintained as memory cells. These data demonstrate that BRM cells are an important component of immunity to respiratory viruses such as influenza virus and suggest that vaccines designed to elicit BRM cells must deliver antigen to the lungs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Halliley, J. L. et al. Long-lived plasma cells are contained within the CD19−CD38hiCD138+ subset in human bone marrow. Immunity 43, 132–145 (2015).
Dorner, T. & Radbruch, A. Antibodies and B cell memory in viral immunity. Immunity 27, 384–392 (2007).
Phan, T. G. & Tangye, S. G. Memory B cells: total recall. Curr. Opin. Immunol. 45, 132–140 (2017).
Bannard, O. & Cyster, J. G. Germinal centers: programmed for affinity maturation and antibody diversification. Curr. Opin. Immunol. 45, 21–30 (2017).
Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247, 52–63 (2012).
Schittek, B. & Rajewsky, K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature 346, 749–751 (1990).
Weisel, F. J., Zuccarino-Catania, G. V., Chikina, M. & Shlomchik, M. J. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 44, 116–130 (2016).
Jaimes, M. C. et al. Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J. Virol. 78, 10967–10976 (2004).
Onodera, T. et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 109, 2485–2490 (2012).
Joo, H. M., He, Y., Sundararajan, A., Huan, L. & Sangster, M. Y. Quantitative analysis of influenza virus-specific B cell memory generated by different routes of inactivated virus vaccination. Vaccine 28, 2186–2194 (2010).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Dogan, I. et al. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 10, 1292–1299 (2009).
Pape, K. A., Taylor, J. J., Maul, R. W., Gearhart, P. J. & Jenkins, M. K. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331, 1203–1207 (2011).
Palladino, G., Mozdzanowska, K., Washko, G. & Gerhard, W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 69, 2075–2081 (1995).
Tomayko, M. M., Steinel, N. C., Anderson, S. M. & Shlomchik, M. J. Cutting edge: hierarchy of maturity of murine memory B cell subsets. J. Immunol. 185, 7146–7150 (2010).
Bernasconi, N. L., Traggiai, E. & Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202 (2002).
Lee, F. E. et al. Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect. J. Immunol. 186, 5514–5521 (2011).
Adachi, Y. et al. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J. Exp. Med. 212, 1709–1723 (2015).
Moyron-Quiroz, J. E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Hwang, J. Y., Randall, T. D. & Silva-Sanchez, A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front. Immunol. 7, 258 (2016).
Taylor, J. J., Pape, K. A. & Jenkins, M. K. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 209, 597–606 (2012).
Zaid, A. et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. USA 111, 5307–5312 (2014).
McMaster, S. R. et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. 11, 1071–1078 (2018).
Moyron-Quiroz, J. E. et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25, 643–654 (2006).
Vu Van, D. et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat. Commun. 7, 10875 (2016).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Keating, R. et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat. Immunol. 14, 1266–1276 (2013).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Misulovin, Z., Yang, X. W., Yu, W., Heintz, N. & Meffre, E. A rapid method for targeted modification and screening of recombinant bacterial artificial chromosome. J. Immunol. Methods 257, 99–105 (2001).
Beckett, D., Kovaleva, E. & Schatz, P. J. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8, 921–929 (1999).
Acknowledgements
The authors would like to thank U. Mudunuru and S. Simpler for animal husbandry and the Rheumatic Diseases Core Center flow cytometry facility, which is supported by AI078907. This work was supported by NIH grants HL69409, AI100127, AI097357, AI109962 to T.D.R. and AI120508 to S.R.A.
Author information
Authors and Affiliations
Contributions
S.R.A., F.E.L. and T.D.R. designed the experiments. J.E.B., B.A.G. and T.D.R. designed the recombinant influenza proteins and J.E.B. and B.A.G. expressed, purified and characterized the tetramers. U.M. and S.R.A performed the surgeries. M.D.S. performed the intratracheal infections. S.R.A performed and analyzed the experiments and generated the figures. S.R.A. and T.D.R. wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Fig. 1 Gating strategies.
Gates are shown sequentially from left to right. Coloured gates are used in the figures listed to the right in font of the same colour.
Supplementary Fig. 2 Identification of influenza-specific memory B cells.
(a–f) Cells from the lungs (a,d), mLNs (b,e) and spleens (c,f) of naïve mice or PR8-infected mice were gated on live, singlet lymphocytes and then on CD19+CD38hiIgD–IgM+ (IgM memory) or CD19+CD38hiIgD–IgM– (ISW memory) (Supplementary Fig. 1c) and analysed for NP-specific or HA-specific memory B cells on day 30 (a–c) or day 70 (d–f) after infection. Data are representative of four experiments with five mice/timepoint.
Supplementary Fig. 3 Phenotypic characteristics of non-circulating and total memory B cells.
(a,b) Mice were infected with PR8 influenza A virus, and cells from the mLN, lung and spleen were gated on live singlet lymphocytes and then on NP-specific CD19+CD38+IgM–IgD– ISW memory B cells that were either B220+/– (combined circulating and non-circulating; a) or B220– (non-circulating; b) (gating strategy as in Fig. 2a–d). The phenotype within these populations was determined as in Fig. 2e–i. Data are representative of five experiments with five mice each. Graphs show individual data points (n = 5) as well as mean ± SD. Data were analysed by one-way ANOVA with Tukey’s correction for multiple comparisons: (a) CD73+PD-L2+ ***p = 0.0002, ****p = 0.0001; (a) CD80–PD-L2+ ****p = 0.0001, ####p = 0.0001; (a) CD69+CD103– ns = 0.4447, *p = 0.0129; (a) CD62L+CD69+ ns = 0.0511, *p = 0.0237; (a) CXCR3+ *p = 0.0350, **p = 0.0054; (b) CD73+PD-L2+ ****p = 0.0001, ####p = 0.0001; (b) CD80–PD-L2+ ****p = 0.0001, ####p = 0.0001; (b) CD69+CD103– ***p = 0.0002, *p = 0.0105; (b) CD62L+CD69+ *p = 0.0471, **p = 0.0042; (b) CXCR3+ *p = 0.0206, ***p = 0.0008; p < 0.05 is considered significant. (c–e) Cells from the mLN (c), spleen (d) and lung (e) of day 44 PR8-infected mice were gated on live, singlet lymphocytes (Supplementary Fig. 1a) and then on NP-specific CD19+CD38+IgD– memory B cells, and the frequency of IgM-, IgA-, IgG1-, IgG2b-, IgG2c- and IgG3-expressing cells was determined. Data are representative of three experiments with five mice.
Supplementary Fig. 4 Identification of influenza-specific, non-circulating and total memory B cells.
(a–l) Mice were infected on day 0, surgically paired with partner mice on day 44 and analysed on day 59. Cells from the lung (a–d), mLN (e–h) or spleen (i–l) were gated on live, singlet lymphocytes (Supplementary Fig. 1a) and subsequently gated on CD19+CD38+IgD–IgM+ (IgM) or CD19+CD38+IgD–IgM– (ISW) NP-specific memory B cells in either the B220– (non-circulating; a,c,e,g,i,k) or B220+/– (combined circulating and non-circulating; b,d,f,h,j,l) fractions. The numbers of NP-specific memory B cells derived from host and partner mice in the lung (c,d), mLN (g,h) and spleen (i,j) are shown for both the non-circulating fraction and the total (combined circulating and non-circulating) fraction. Data are representative of three experiments combined, totalling 11 pairs of mice. Graphs show individual data as well as mean ± SD. Significance was determined using one-way ANOVA followed by the Bonferroni–Sidak method for multiple comparisons: (c) Lung, IgM HA B220– **p = 0.0031, *p = 0.0243; (c) Lung, IgM NP B220– ****p = 0.0001, **p = 0.0040; (c) Lung, ISW HA B220– ***p = 0.0003, ###p = 0.0002; (c) Lung, ISW NP B220– ****p = 0.0001, **p = 0.0022; (d) Lung, IgM HA B220+/– **p = 0.0013, *p = 0.0158; (d) Lung, IgM NP B220+/– ****p = 0.0001, **p = 0.0017; (d) Lung, ISW HA B220+/– ****p = 0.0001, ***p = 0.0001; (d) Lung, ISW NP B220+/– ****P = 0.0001, **p = 0.0024; (g) mLN, IgM HA B220– p = 0.0618, p = 0.1136; (g) mLN, IgM NP B220– p = 0.0783, *p = 0.0183; (g) mLN, ISW HA B220– **p = 0.0014, ****p = 0.0001; (g) mLN, ISW NP B220– **p = 0.0079, ****p = 0.0001; (h) mLN, IgM HA B220+/– p = 0.0718, p = 0.1631; (h) mLN, IgM NP B220+/– p = 0.0746, *p = 0.0237; (h) mLN, ISW HA B220+/– **p = 0.0022, ****p = 0.0001; (h) mLN, ISW NP B220+/– **p = 0.0081, ***p = 0.0001; (k) Spleen, IgM HA B220– p = 0.2821, **p = 0.0039; (k) Spleen, IgM NP B220– *p = 0.0160, **p = 0.0012; (k) Spleen, ISW HA B220– p = 0.6749, p = 0.2475; (k) Spleen, ISW NP B220– *p = 0.0337, **p = 0.0028; (l) Spleen, IgM HA B220+/– p = 0.0662, *p = 0.0116; (l) Spleen, IgM NP B220+/– **p = 0.0078, *p = 0.0156; (l) Spleen, ISW HA B220+/– p = 0.4171, p = 0.1469; (l) Spleen, ISW NP B220+/– **p = 0.0029, *p = 0.0108; p < 0.05 is considered significant.
Supplementary Fig. 5 HA-specific BRM cells in the lung are generated from early CD40-dependent precursors.
(a–d) Mice were infected with PR8 and administered anti-CD40L (MR1) or isotype control (CT) antibody every other day for 10 days starting on day 5 (a), day 10 (b), day 20 (c) or day 30 (d). Cells from the lung were gated on live, singlet lymphocyte, B220–CD19+CD38+IgM+IgD– IgM BRM cells or B220–CD19+CD38+IgM–IgD– ISW BRM cells (Supplementary Fig. 1g). Data are representative of three experiments with five mice/group per timepoint. Graphs show mean ± SD as well as individual data points. Significance was determined using an unpaired, two-tailed t-test: ***p = 0.0009, ###p = 0.0006 (a), **p = 0.0090 (b), *p = 0.0219 (c). p < 0.05 is considered significant.
Supplementary Fig. 6 Poor recruitment of antigen-non-specific IgM memory cells to inflamed lungs.
(a–g), Mice were peritoneally infected with PR8 on day 0, intranasally challenged with X31 on day 30 and analysed on day 40 (a–d) and day 75 (e–g). Cells from the lung were gated on live, singlet lymphocytes (Supplementary Fig. 1a) and subsequently gated on CD19+B220–CD38+IgD–IgM+ IgM memory B cells (a). NP-specific (b,e), HA(PR8)-specific (c,f) and HA(X31)-specific (d,g) IgM BRM cells were enumerated on day 40 (b–d) and day 75 (e–g). Graphs show individual data points as well as mean ± SD. These data are representative of two independent experiments with five mice/timepoint. Data were analysed with a one-sided unpaired t-test: ***p = 0.001 (b), ***p = 0.0098 (d), *p = 0.0205 (e). p < 0.05 is considered significant.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6
Rights and permissions
About this article
Cite this article
Allie, S.R., Bradley, J.E., Mudunuru, U. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 20, 97–108 (2019). https://doi.org/10.1038/s41590-018-0260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0260-6
This article is cited by
-
B cell memory: from generation to reactivation: a multipronged defense wall against pathogens
Cell Death Discovery (2024)
-
Distribution of multi-level B cell subsets in thymoma and thymoma-associated myasthenia gravis
Scientific Reports (2024)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Tissue-resident B cells orchestrate macrophage polarisation and function
Nature Communications (2023)
-
Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA
Nature Biomedical Engineering (2023)