Abstract
The mechanisms by which the sensitivity of naive CD4+ T cells to stimulation by the cognate antigen via the T cell antigen receptor (TCR) determines their differentiation into distinct helper T cell subsets remain elusive. Here we demonstrate functional collaboration of the ubiquitin E3 ligases Itch and WWP2 in regulating the strength of the TCR signal. Mice lacking both Itch and WWP2 in T cells showed spontaneous autoimmunity and lung inflammation. CD4+ T cells deficient in Itch and WWP2 exhibited hypo-responsiveness to TCR stimulation and a bias toward differentiation into the TH2 subset of helper T cells. Itch and WWP2 formed a complex and cooperated to enhance TCR-proximal signaling by catalyzing the conjugation of atypical ubiquitin chains to the phosphatase SHP-1 and reducing the association of SHP-1 with the tyrosine kinase Lck. These findings indicate that targeted ubiquitination regulates the strength of the TCR signal and differentiation toward the TH2 lineage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhu, J. & Paul, W. E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238, 247–262 (2010).
Ho, I. C., Tai, T. S. & Pai, S. Y. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 9, 125–135 (2009).
Constant, S., Pfeiffer, C., Woodard, A., Pasqualini, T. & Bottomly, K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182, 1591–1596 (1995).
Yamane, H. & Paul, W. E. Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunol. Rev. 252, 12–23 (2013).
Yamane, H., Zhu, J. & Paul, W. E. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 202, 793–804 (2005).
Fang, D. et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3, 281–287 (2002).
Heissmeyer, V. et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5, 255–265 (2004).
Yang, B. et al. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat. Immunol. 9, 1356–1363 (2008).
Rotin, D. & Kumar, S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 (2009).
Aki, D., Zhang, W. & Liu, Y. C. The E3 ligase Itch in immune regulation and beyond. Immunol. Rev. 266, 6–26 (2015).
Chen, A. et al. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell Biol. 29, 5348–5356 (2009).
Hustad, C. M. et al. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics 140, 255–265 (1995).
Lohr, N. J. et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am. J. Hum. Genet. 86, 447–453 (2010).
Yang, Y. et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 110, 5115–5120 (2013).
Angers, A., Ramjaun, A. R. & McPherson, P. S. The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. J. Biol. Chem. 279, 11471–11479 (2004).
Martin-Serrano, J., Eastman, S. W., Chung, W. & Bieniasz, P. D. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell Biol. 168, 89–101 (2005).
Sasaki, T. et al. Genome-wide gene expression profiling revealed a critical role for GATA3 in the maintenance of the Th2 cell identity. PLoS One 8, e66468 (2013).
Zhu, J., Cote-Sierra, J., Guo, L. & Paul, W. E. Stat5 activation plays a critical role in Th2 differentiation. Immunity 19, 739–748 (2003).
Naramura, M. et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 3, 1192–1199 (2002).
Johnson, D. J. et al. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J. Exp. Med. 210, 1419–1431 (2013).
Lorenz, U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 (2009).
Stefanová, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 4, 248–254 (2003).
Paul, W. E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235 (2010).
Constant, S. L. & Bottomly, K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15, 297–322 (1997).
Tubo, N. J. & Jenkins, M. K. TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol. 35, 591–596 (2014).
van Panhuys, N., Klauschen, F. & Germain, R. N. T-cell-receptor-dependent signal intensity dominantly controls CD4+ T cell polarization in vivo. Immunity 41, 63–74 (2014).
Jorritsma, P. J., Brogdon, J. L. & Bottomly, K. Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J. Immunol. 170, 2427–2434 (2003).
Shultz, L. D. et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73, 1445–1454 (1993).
Tsui, H. W., Siminovitch, K. A., de Souza, L. & Tsui, F. W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 4, 124–129 (1993).
Salmond, R. J., Filby, A., Qureshi, I., Caserta, S. & Zamoyska, R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 228, 9–22 (2009).
Choi, S. et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat. Immunol. 18, 433–441 (2017).
Fu, G. et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 504, 441–445 (2013).
Paster, W. et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 34, 393–409 (2015).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Wang, Q. et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 (2014).
Harreman, M. et al. Distinct ubiquitin ligases act sequentially for RNA polymerase II polyubiquitylation. Proc. Natl. Acad. Sci. USA 106, 20705–20710 (2009).
Hwang, C. S., Shemorry, A., Auerbach, D. & Varshavsky, A. The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat. Cell Biol. 12, 1177–1185 (2010).
Scott, D. C. et al. Two distinct types of E3 ligases work in unison to regulate substrate ubiquitylation. Cell 166, 1198–1214 (2016).
Xiao, N. et al. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 15, 657–666 (2014).
Zou, W. et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat. Cell Biol. 13, 59–65 (2011).
Wei, W., Li, M., Wang, J., Nie, F. & Li, L. The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol. Cell Biol. 32, 3903–3912 (2012).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Martin, D. A. et al. Autoimmunity stimulated by adoptively transferred dendritic cells is initiated by both αβ and γδ T cells but does not require MyD88 signaling. J. Immunol. 179, 5819–5828 (2007).
Mihara, M. et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J. Clin. Invest. 106, 91–101 (2000).
Huang, H. et al. K33-linked polyubiquitination of T cell receptor-ζ regulates proteolysis-independent T cell signaling. Immunity 33, 60–70 (2010).
Couture, C. et al. Activation of p56lck by p72syk through physical association and N-terminal tyrosine phosphorylation. Mol. Cell Biol. 14, 5249–5258 (1994).
Myers, M. D., Dragone, L. L. & Weiss, A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRζ for degradation. J. Cell Biol. 170, 285–294 (2005).
Acknowledgements
We thank L.G. Glimcher (Dana-Farber Cancer Institute) for the Wwp2−/− mouse strain; H. Deng for mass spectrometry; Z. Yang for purification of glutathione S-transferase fusion proteins; H. Li for imaging analysis, J. Greenbaum, A. Sethi and G. Tian for RNA-seq analysis; and members of the Liu laboratory for discussions. Supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research (fellowship to D.A.), the National Natural Science Foundation of China (81725010 and XBD19000000 to W. Zou), the Ministry of Science and Technology of China (YFA0505802, YFC0903900, NSFC81630041), the US National Institutes of Health (RO1AI123398 and R21AI122258) and the Tsinghua-Peking Center for Life Sciences (Y.-C.L.).
Author information
Authors and Affiliations
Contributions
D.A., H.L., and W. Zhang performed experiments and analyzed data; M.Z., C.E. and J.H.L. prepared reagents and maintained mouse breeding colonies; W. Zou provided the Wwp2−/− mouse strain and performed initial proteomics analysis; and D.A. and Y.-C.L. designed the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
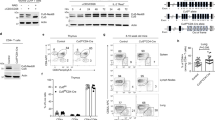
Supplementary Figure 1 Itch forms complex with WWP2.
(a) Schematic representation of mouse Itch and WWP2 protein showing functional domains and amino acid sequence similarity. (b) Immunoblot of precipitates from HEK293T cells transfected with the indicated expression vectors, then lysed and immunoprecipitated with anti-Myc antibody. (c) Interaction of Itch and WWP2 in Jurkat T cells co-transfected with Xpress-Itch and FLAG-WWP2 expression vectors, stimulated with anti-CD3 for 15 min, lysed and then immunoprecipitated with anti-Xpress antibody. Data are representative of two independent experiments.
Supplementary Figure 2 Itch and WWP2 cooperatively protect against autoimmunity.
(a) Red blood cell counts (left) and hemoglobin concentration (right) in the peripheral blood from 6-8 wks old WT and DKO mice. Data are means ± SD (n=8 per group). (b) Productions of anti-dsDNA (left) or anti-histone antibodies (right) in the serum from WT or DKO bone marrow chimeric mice determined by ELISA. Data are means ± SD (n=6 per group). (c) The lung sections from WT or DKO bone marrow chimeric mice stained with hematoxylin and eosin. (d) Frequencies (left) or cell numbers (right) of eosinophils (EOS), macrophages (MAC), dendritic cells (DC) and neutrophils (NEU) in the lung from bone marrow chimeric mice in Fig. 2e. Data are means ± SD (EOS, MAC, NEU; n=8, DC; n=5 per group). (e) Productions of multiple Ig subclasses in the serum from WT or DKO bone marrow chimeric mice determined by ELISA. Data are means ± SD (n=10 per group). Each symbol represents individual mouse. Data are pooled from or representative of two to three independent experiments. P values were calculated by unpaired two-tailed Student’s t test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; NS, non-significant.
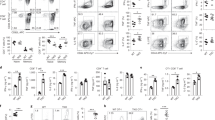
Supplementary Figure 3 Cooperation of Itch and WWP2 in TH2 differentiation.
(a) Frequencies of memory-like and naive CD4+ T cells in the spleen from WT and DKO bone marrow chimeric mice. Data are means ± SD (n=9 per group). (b) Representative plots of CD4+Foxp3+ T cells in the spleen. (c) Relative expressions of genes encoding cytokines and transcription factors in WT versus WWP2-/-, Itchf/fCd4-Cre, or DKO CD4+ T cells. (d) Relative expressions of genes encoding selected cytokines and transcription factors from splenic memory-like CD4+ T cells from WT and DKO bone marrow chimeric mice stimulated with anti-CD3+CD28 for 4 h. The mRNA expression levels were normalized to beta-Actin. Data are means ± SD (n=3 per group). (e) Expression of selected TH2 specific genes in PMA+ionomycin stimulated WT, WWP2-/-, Itchf/fCd4-Cre, or DKO CD4+ T cells presented as log2RPKM values. (f) Representative plots of IL-4+ or IFNgamma+ CD4+ T cells in Fig. 3c. (g) IL-4 production by sorted naïve CD4+ T cells stimulated with anti-CD3+CD28 for 48 h and determined by ELISA. Data are means ± SD (n=4 per group). (h) Expression of genes encoding selected STAT5 targets in CD4+ T cells stimulated with PMA and Ionomycin presented as log2RPKM values. Data are pooled from or representative of two to five independent experiments. P values were calculated by unpaired two-tailed Student’s t test (a, d, g) or one-way ANOVA with Bonferroni’s multiple comparison test (c). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; NS, non-significant.
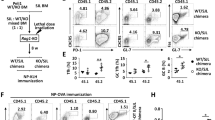
Supplementary Figure 4 Itch and WWP2 control TCR-proximal signaling.
(a) IL-2 production by sorted CD4+CD45.2+ T cells from WT or DKO bone marrow chimeric mice stimulated with anti-CD3+CD28 for 48 h and determined by ELISA. Data are means ± SD (n=3 per group). (b) Schematic representation of genome-editing strategy to delete endogenous Itch and WWP2 in Jurkat T cells. The single-guide (sg) RNA sequences target within exon 4 in hItch and exon 3 in hWwp2 and depicted in red. The protospacer adjacent motifs are depicted in blue. (c) Immunoblot of the cell lysates from WT and DKO Jurkat T cells with the indicated antibodies. (d) Internalized TCRbeta in anti-CD3+CD28 stimulated CD4+CD45.2+ T cells from bone marrow chimeric mice analyzed at the indicated time by flow cytometry. Data are means ± SD (n=3 per group). (e) Recycled TCRbeta in anti-CD3+CD28 stimulated CD4+CD45.2+ T cells from bone marrow chimeric mice analyzed at the indicated time by flow cytometry. Data are means ± SD (n=3 per group). Data are pooled from or representative of two to three independent experiments. P values were calculated by unpaired two-tailed Student’s t test. **p<0.01.
Supplementary Figure 5 SHP-1 is a common substrate for Itch and WWP2.
(a,b) Immunoblot of precipitates from HEK293T cells transfected with FLAG-SHP-1 and Itch (a) or WWP2 (b), then lysed and immunoprecipitaed with anti-FLAG antibody. (c) Immunoblot of precipitates from HEK293T cells transfected with the indicated expression vectors, lysed and immunoprecipitated with anti-FLAG antibody. (d) Immunoblot of HEK293T cells transfected with the indicated siRNA, subsequently transfected with FLAG-SHP-1 and HA-Ubiquitin expression vectors. Data are representative of two independent experiments.
Supplementary Figure 6 Identification of ubiquitination sites of mouse SHP-1 by Itch and WWP2.
Amino acid sequence alignment of SHP-1 around the identified lysine residues as the ubiquitination sites among the indicated species.
Supplementary Figure 7 SHP-1 ubiquitination does not affect its protein stability or phosphatase activity, nor thymic development.
(a) Immunoblot of cell lysates from WT and DKO Jurkat T cells treated with 10 μM MG132 for the indicated time points and analyzed by immunoblotting with the indicated antibodies. (b) immunoblot of cell lysates from Jurkat cells expressing FLAG-WT-SHP-1 and FLAG-3KR-SHP-1 treated with 10 μM MG132 for the indicated time points and analyzed by immunoblotting with the indicated antibodies. (c) In vitro PTP assay of GST-WT-SHP-1 or GST-3KR-SHP-1 measured using DiFMUP as the substrate. Data are presented as mean ± SEM of fluorescence signal with technical replicates (n=3). Right, CBB staining of each purified protein as an input control. (d,e) Immunoblot of precipitates from Jurkat T cells transfected with FLAG-WT-SHP-1 or FLAG-3KR-SHP-1, lysed and immunopreciptated with anti-FLAG antibody. (f) Immunoblot of cell lysates from WT and DKO Jurkat T cells analyzed by immunoblotting with the indicated antibodies. (g) Frequencies of CD4-CD8-, CD4+CD8+, CD4+CD8- and CD4-CD8+ subsets in GFP+ thymocytes from bone marrow chimeric mice reconstituted with bone marrow cells transduced with retrovirus expression vector for WT- or 3KR-SHP-1. Right, representative plots of these subsets. Data are presented as mean ± SD (n=7). (h) Frequencies of CD4+CD69+ subset in GFP+ thymocytes from WT- or 3KR-SHP-1 bone marrow chimeric mice. Right, representative plots of this subset. Data are presented as mean ± SD (n=7). (i) Frequencies of CD45.1+ (WT) or CD45.2+ (DKO) cells in the thymocytes of mixed bone marrow chimeric mice. Data are presented as mean ± SD (n=3). (j) Frequencies of CD4-CD8-, CD4+CD8+, CD4+CD8- and CD4-CD8+ subsets in CD45.1+ (WT) or CD45.2+ (DKO) thymocytes of mixed bone marrow chimeric mice. Right, representative plots of these subsets. Data are presented as mean ± SD (n=3). (k) Frequencies of CD4+CD69+ subset in CD45.1+ (WT) or CD45.2+ (DKO) thymocytes of mixed bone marrow chimeric mice. Data are presented as mean ± SD (n=3). (l) Frequencies of splenic naïve (CD25-CD44-CD62Lhi) (left), and memory-like (CD44hiCD62L-) (middle) GFP+CD4+ T cells from WT- or 3KR-SHP-1 bone marrow chimeric mice. Right, representative plots of these subsets. Data are presented as mean ± SD (n=7). Data are representative of two to five independent experiments. P values were calculated by unpaired two-tailed Student’s t test. NS, non-significant.
Supplementary information
Supplementary Figures
Supplementary Figures 1–7 and Supplementary Tables 1–4
Supplementary Data Set 1
unprocessed gel image
Rights and permissions
About this article
Cite this article
Aki, D., Li, H., Zhang, W. et al. The E3 ligases Itch and WWP2 cooperate to limit TH2 differentiation by enhancing signaling through the TCR. Nat Immunol 19, 766–775 (2018). https://doi.org/10.1038/s41590-018-0137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0137-8
This article is cited by
-
Down-regulation of WWP2 aggravates Type 2 diabetes mellitus-induced vascular endothelial injury through modulating ubiquitination and degradation of DDX3X
Cardiovascular Diabetology (2023)
-
HIV-1 Vif suppresses antiviral immunity by targeting STING
Cellular & Molecular Immunology (2022)
-
Selective targeting of ubiquitination and degradation of PARP1 by E3 ubiquitin ligase WWP2 regulates isoproterenol-induced cardiac remodeling
Cell Death & Differentiation (2020)
-
A multi-lock inhibitory mechanism for fine-tuning enzyme activities of the HECT family E3 ligases
Nature Communications (2019)
-
Wwp2 maintains cartilage homeostasis through regulation of Adamts5
Nature Communications (2019)