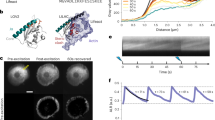
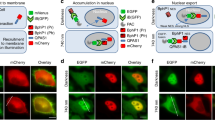
Abstract
Here we introduce Z-lock, an optogenetic approach for reversible, light-controlled steric inhibition of protein active sites. The light oxygen voltage (LOV) domain and Zdk, a small protein that binds LOV selectively in the dark, are appended to the protein of interest where they sterically block the active site. Irradiation causes LOV to change conformation and release Zdk, exposing the active site. Computer-assisted protein design was used to optimize linkers and Zdk-LOV affinity, for both effective binding in the dark, and effective light-induced release of the intramolecular interaction. Z-lock cofilin was shown to have actin severing ability in vitro, and in living cancer cells it produced protrusions and invadopodia. An active fragment of the tubulin acetylase αTAT was similarly modified and shown to acetylate tubulin on irradiation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
Code availability
Code is available from the authors upon request or at http://www.hahnlab.com.
Change history
21 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Bravo-Cordero, J. J., Magalhaes, M. A. O., Eddy, R. J., Hodgson, L. & Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 14, 405–415 (2013).
Sidani, M. et al. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J. Cell Biol. 179, 777–791 (2007).
Zoncu, R. et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl Acad. Sci. USA 104, 3793–3798 (2007).
Oser, M. & Condeelis, J. The cofilin activity cycle in lamellipodia and invadopodia. J. Cell Biochem. 108, 1252–1262 (2009).
DesMarais, V., Ghosh, M., Eddy, R. & Condeelis, J. Cofilin takes the lead. J. Cell Sci. 118, 19–26 (2005).
Chen, Q. & Pollard, T. D. Actin filament severing by cofilin dismantles actin patches and produces mother filaments for new patches. Curr. Biol. 23, 1154–1162 (2013).
Yang, N. et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393, 809–812 (1998).
Aizawa, H., Sutoh, K. & Yahara, I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in dictyostelium. J. Cell Biol. 132, 335–344 (1996).
Aizawa, H. et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 4, 367–373 (2001).
Ghosh, M. et al. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 304, 743–746 (2004).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Hughes, R. M. & Lawrence, D. S. Optogenetic engineering: light-directed cell motility. Angew. Chem. Int. Ed. Engl. 53, 10904–10907 (2014).
Courtemanche, N., Pollard, T. D. & Chen, Q. Avoiding artefacts when counting polymerized actin in live cells with LifeAct fused to fluorescent proteins. Nat. Cell Biol. 18, 676–683 (2016).
Dagliyan, O. et al. Engineering extrinsic disorder to control protein activity in living cells. Science 354, 1441–1444 (2016).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Hayashi-Takagi, A. et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338 (2015).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Strickland, D., Moffat, K. & Sosnick, T. R. Light-activated DNA binding in a designed allosteric protein. Proc. Natl Acad. Sci. USA 105, 10709–10714 (2008).
Yi, J. J., Wang, H., Vilela, M., Danuser, G. & Hahn, K. M. Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides. ACS Synth. Biol. 3, 788–795 (2014).
Weitzman, M. & Hahn, K. M. Optogenetic approaches to cell migration and beyond. Curr. Opin. Cell Biol. 30, 112–120 (2014).
Wang, X., Chen, X. & Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 9, 266–269 (2012).
Zoltowski, B. D., Vaccaro, B. & Crane, B. R. Mechanism-based tuning of a LOV domain photoreceptor. Nat. Chem. Biol. 5, 827–834 (2009).
Kawano, F., Aono, Y., Suzuki, H. & Sato, M. Fluorescence imaging-based high-throughput screening of fast- and slow-cycling LOV proteins. PLoS One 8, e82693 (2013).
Wang, H. et al. LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat. Methods 13, 755–758 (2016).
Wang, H. & Hahn, K. M. LOVTRAP: a versatile method to control protein function with light. Curr. Protoc. Cell Biol. 73, 21.10.1–21.10.14 (2016).
Yu, Y. & Lutz, S. Circular permutation: a different way to engineer enzyme structure and function. Trends Biotechnol. 29, 18–25 (2011).
Zhou, X. X., Chung, H. K., Lam, A. J. & Lin, M. Z. Optical control of protein activity by fluorescent protein domains. Science 338, 810–814 (2012).
Zhou, X. X., Fan, L. Z., Li, P., Shen, K. & Lin, M. Z. Optical control of cell signaling by single-chain photoswitchable kinases. Science 355, 836–842 (2017).
Lai, F. P. L. et al. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 27, 982–992 (2008).
Pope, B. & Weeds, A. G. Binding of pig plasma gelsolin to F-actin and partial fractionation into calcium-dependent and calcium-independent forms. Eur. J. Biochem. 161, 85–93 (1986).
Huang, P.-S. et al. RosettaRemodel: a generalized framework for flexible backbone protein design. PLoS One 6, e24109 (2011).
Kellogg, E. H., Leaver-Fay, A. & Baker, D. Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins 79, 830–838 (2011).
Chan, A. Y., Bailly, M., Zebda, N., Segall, J. E. & Condeelis, J. S. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J. Cell Biol. 148, 531–542 (2000).
Neri, A., Welch, D., Kawaguchi, T. & Nicolson, G. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J. Natl. Cancer Inst. 68, 507–517 (1982).
Chan, A. Y. et al. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell Sci. 111(Pt 2), 199–211 (1998).
Eddy, R. J., Weidmann, M. D., Sharma, V. P. & Condeelis, J. S. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 27, 595–607 (2017).
Yamaguchi, H. et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 168, 441–452 (2005).
Oser, M. et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571–587 (2009).
Beaty, B. T. et al. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol. Biol. Cell 24, 1661–75, S1 (2013).
Haggarty, S. J., Koeller, K. M., Wong, J. C., Grozinger, C. M. & Schreiber, S. L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl Acad. Sci. USA 100, 4389–4394 (2003).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
North, B. J., Marshall, B. L., Borra, M. T., Denu, J. M. & Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 (2003).
Miotto, B. & Struhl, K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by geminin. Mol. Cell 37, 57–66 (2010).
Borra, M. T., Langer, M. R., Slama, J. T. & Denu, J. M. Substrate specificity and kinetic mechanism of the Sir2 family of NAD+-dependent histone/protein deacetylases. Biochemistry 43, 9877–9887 (2004).
Shida, T., Cueva, J. G., Xu, Z., Goodman, M. B. & Nachury, M. V. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl Acad. Sci. USA 107, 21517–21522 (2010).
Friedmann, D. R., Aguilar, A., Fan, J., Nachury, M. V. & Marmorstein, R. Structure of the α-tubulin acetyltransferase, αTAT1, and implications for tubulin-specific acetylation. Proc. Natl Acad. Sci. USA 109, 19655–19660 (2012).
Szyk, A. et al. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405–1415 (2014).
Leaver-Fay, A. et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Meth. Enzymol. 487, 545–574 (2011).
Kuhlman, B., Jacobs, T. & Linskey, T. Computational design of protein linkers. Methods Mol. Biol. 1414, 341–351 (2016).
Suhre, K. & Sanejouand, Y.-H. ElNemo: a normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res. 32, W610–W614 (2004).
Li, T. et al. Incorporation of DDR2 clusters into collagen matrix via integrin-dependent posterior remnant tethering. Int. J. Biol. Sci. 14, 654–666 (2018).
Sharma, V. P. et al. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Curr. Biol. 23, 2079–2089 (2013).
Leibovitz, A. The growth and maintenance of tissue–cell cultures in free gas exchange with the atmosphere. Am. J. Epidemiol. 78, 173–180 (1963).
Sharma, V. P., Entenberg, D. & Condeelis, J. High-resolution live-cell imaging and time-lapse microscopy of invadopodium dynamics and tracking analysis. Methods Mol. Biol. 1046, 343–357 (2013).
Hodgson, L., Shen, F. & Hahn, K. Biosensors for characterizing the dynamics of rho family GTPases in living cells. Curr. Protoc. Cell Biol. Chapter 14, Unit 14.11.1–14.11.26 (2010).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 9, 62–66 (1979).
Klejnot, M. et al. Analysis of the human cofilin 1 structure reveals conformational changes required for actin binding. Acta Crystallogr. D 69, 1780–1788 (2013).
Acknowledgements
We thank the NIH for supporting this work (grant no. GM122596 to K.M.H., grant nos. CA150344 and CA216248 to J.S.C.). O.J.S. is a recipient of a Ruth L. Kirschstein National Research Service Award (no. 1F31CA192739). We thank M. Azoitei for assisting with Rosetta modeling, T. Watanabe for assistance with localized photoactivation experiments and C. Onyeji for help with cloning and biochemical assays. N.P. was supported by grant PF-16-18-01-CSM from the American Cancer Society.
Author information
Authors and Affiliations
Contributions
J.S.C. and K.M.H. conceived of the project. O.J.S., B.L. and N.P. carried out the experiments, with assistance and advice from V.P.S. and R.J.E. in live cell imaging and assay of effects on cofilin. K.M.H. and H.W. conceived of Z-lock, and J.S.C. initiated the focus on cofilin. K.M.H. and J.S.C. supervised the project. O.J.S., F.D.T. and B.K. performed Rosetta modeling. A.T.P. purified proteins. O.J.S. and K.M.H. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–2 and Supplementary Figures 1–13.
Supplementary Video 1
Original images of blots and stain-free gels used for Fig. 4b.
Supplementary Video 2
Photoactivation of Z-lock cofilin leads to protrusion.
Rights and permissions
About this article
Cite this article
Stone, O.J., Pankow, N., Liu, B. et al. Optogenetic control of cofilin and αTAT in living cells using Z-lock. Nat Chem Biol 15, 1183–1190 (2019). https://doi.org/10.1038/s41589-019-0405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0405-4
This article is cited by
-
A guide to the optogenetic regulation of endogenous molecules
Nature Methods (2021)
-
Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination
Nature Communications (2021)
-
Tools of the trade: studying actin in zebrafish
Histochemistry and Cell Biology (2020)
-
Cutting actin with light
Nature Chemical Biology (2019)