Abstract
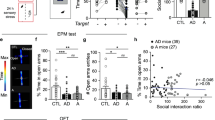
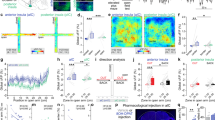
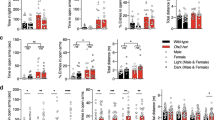
Iron is essential for a broad range of biochemical processes in the brain, but the mechanisms of iron metabolism in the brain remain elusive. Here we show that iron functionally translocates among brain regions along specific axonal projections. We identified two pathways for iron transport in the brain: a pathway from ventral hippocampus (vHip) to medial prefrontal cortex (mPFC) to substantia nigra; and a pathway from thalamus (Tha) to amygdala (AMG) to mPFC. While vHip–mPFC transport modulates anxiety-related behaviors, impairment of Tha–AMG–mPFC transport did not. Moreover, vHip–mPFC iron transport is necessary for the behavioral effects of diazepam, a well-known anxiolytic drug. By contrast, genetic or pharmacological promotion of vHip–mPFC transport produced anxiolytic-like effects and restored anxiety-like behaviors induced by repeated restraint stress. Taken together, these findings provide key insights into iron metabolism in the brain and identify the mechanisms underlying iron transport in the brain as a potential target for development of novel anxiety treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hentze, M. W., Muckenthaler, M. U., Galy, B. & Camaschella, C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010).
Crielaard, B. J., Lammers, T. & Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 16, 400–423 (2017).
Rouault, T. A. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 551–564 (2013).
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R. & Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060 (2014).
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5, 863–873 (2004).
Hill, J. M. & Switzer III, R. C. The regional distribution and cellular localization of iron in the rat brain. Neuroscience 11, 595–603 (1984).
Allen, R. P., Barker, P. B., Wehrl, F. W., Song, H. K. & Earley, C. J. MRI measurement of brain iron in patients with restless legs syndrome. Neurology 56, 263–265 (2001).
Moos, T. & Morgan, E. H. Kinetics and distribution of [59Fe–125I]transferrin injected into the ventricular system of the rat. Brain Res. 790, 115–128 (1998).
Hill, J. M., Ruff, M. R., Weber, R. J. & Pert, C. B. Transferrin receptors in rat brain: neuropeptide-like pattern and relationship to iron distribution. Proc. Natl Acad. Sci. USA 82, 4553–4557 (1985).
Moos, T. & Rosengren Nielsen, T. Ferroportin in the postnatal rat brain: implications for axonal transport and neuronal export of iron. Semin. Pediatr. Neurol. 13, 149–157 (2006).
Moos, T., Rosengren Nielsen, T., Skjorringe, T. & Morgan, E. H. Iron trafficking inside the brain. J. Neurochem. 103, 1730–1740 (2007).
Sohn, Y. S., Breuer, W., Munnich, A. & Cabantchik, Z. I. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood 111, 1690–1699 (2008).
Meyer-Lindenberg, A. From maps to mechanisms through neuroimaging of schizophrenia. Nature 468, 194–202 (2010).
Giedd, J. N. & Rapoport, J. L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67, 728–734 (2010).
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 (1999).
Padilla-Coreano, N. et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89, 857–866 (2016).
Lear, J. et al. High-resolution elemental bioimaging of Ca, Mn, Fe, Co, Cu, and Zn employing LA-ICP-MS and hydrogen reaction gas. Anal. Chem. 84, 6707–6714 (2012).
Mala, H. et al. Delayed intensive acquisition training alleviates the lesion-induced place learning deficits after fimbria-fornix transection in the rat. Brain Res. 1445, 40–51 (2012).
Naumann, T. et al. Altered neuronal responses and regulation of neurotrophic proteins in the medial septum following fimbria-fornix transection in CNTF- and leukaemia inhibitory factor-deficient mice. Eur. J. Neurosci. 24, 2223–2232 (2006).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Capelli, P., Pivetta, C., Soledad Esposito, M. & Arber, S. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377 (2017).
Chotiwat, C. & Harris, R. B. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm. Behav. 50, 489–495 (2006).
Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F. & Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 134, 49–57 (2002).
Low, K. et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290, 131–134 (2000).
Gray, J. D. & McEwen, B. S. Lithium’s role in neural plasticity and its implications for mood disorders. Acta Psychiatr. Scand. 128, 347–361 (2013).
Walia, V., Garg, C. & Garg, M. NO–sGC–cGMP signaling influence the anxiolytic like effect of lithium in mice in light and dark box and elevated plus maze. Brain Res. 1704, 114–126 (2019).
Haj-Mirzaian, A. et al. Lithium attenuated the depressant and anxiogenic effect of juvenile social stress through mitigating the negative impact of interlukin-1β and nitric oxide on hypothalamic–pituitary–adrenal axis function. Neuroscience 315, 271–285 (2016).
Lei, P. et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol. Psychiatry 22, 396–406 (2017).
Carlen, M. What constitutes the prefrontal cortex? Science 358, 478–482 (2017).
Oner, P., Oner, O., Azik, F. M., Cop, E. & Munir, K. M. Ferritin and hyperactivity ratings in attention deficit hyperactivity disorder. Pediatr. Int. 54, 688–692 (2012).
Cortese, S., Angriman, M., Lecendreux, M. & Konofal, E. Iron and attention deficit/hyperactivity disorder: what is the empirical evidence so far? A systematic review of the literature. Expert Rev. Neurother. 12, 1227–1240 (2012).
Dexter, D. T. et al. Increased nigral iron content in postmortem Parkinsonian brain. Lancet 2, 1219–1220 (1987).
Texel, S. J. et al. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. J. Neurochem. 120, 125–134 (2012).
Beard, J. L., Erikson, K. M. & Jones, B. C. Neurobehavioral analysis of developmental iron deficiency in rats. Behav. Brain Res. 134, 517–524 (2002).
Kim, J. & Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 25, 1101–1107 (2014).
Pellegrino, R. M. et al. Transferrin receptor 2 dependent alterations of brain iron metabolism affect anxiety circuits in the mouse. Sci. Rep. 6, 30725 (2016).
Maaroufi, K. et al. Impairment of emotional behavior and spatial learning in adult Wistar rats by ferrous sulfate. Physiol. Behav. 96, 343–349 (2009).
Stremmel, W., Lotz, G., Niederau, C., Teschke, R. & Strohmeyer, G. Iron uptake by rat duodenal microvillous membrane vesicles: evidence for a carrier mediated transport system. Eur. J. Clin. Invest. 17, 136–145 (1987).
Cao, X. et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777 (2013).
Zhu, X. H. et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J. Neurosci. 30, 12653–12663 (2010).
Moos, T., Bernth, N., Courtois, Y. & Morgan, E. H. Developmental iron uptake and axonal transport in the retina of the rat. Mol. Cell. Neurosci. 46, 607–613 (2011).
Guo, Y. Y. et al. Acute stress induces down-regulation of large-conductance Ca2+-activated potassium channels in the lateral amygdala. J. Physiol. 590, 875–886 (2012).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (81930034 and 81325008 to X.H.Z.), the Natural Science Foundation of Guangdong Province (2016A030308005 to X.H.Z.), the Guangzhou Science and Technology Project (201804020061 to X.H.Z.), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_16R37 to X.H.Z.) and Science and Technology Program of Guangdong (2018B030334001 to X.H.Z.). The authors thank the laboratories of Y.-Y. Fang, S.-J. Li and X.-W. Li for their technical support.
Author information
Authors and Affiliations
Contributions
X.H.Z. designed the research. X.H.Z., Z.W., Y.N.Z. and W.C.X. analyzed the data and wrote the paper. Z.W. performed experiments for stereotaxic microinjections, FFT and immunostaining. Z.W., Y.N.Z., P.Y., M.Z.Z. and L.Q.J. conducted the behavioral tests. Y.N.Z. and P.Y. performed experiments for 56Fe detections, quantitative PCR with reverse transcription and immunoblots. Z.W., J.Z.Z. and X.H. performed experiments for 59Fe and 57Fe detections L.Q.J. performed Perl’s staining. M.Z.Z., P.Y. and L.Q.J. performed genotyping.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Supplementary Figures 1–13
Supplementary Dataset
Statistical parameters
Rights and permissions
About this article
Cite this article
Wang, Z., Zeng, YN., Yang, P. et al. Axonal iron transport in the brain modulates anxiety-related behaviors. Nat Chem Biol 15, 1214–1222 (2019). https://doi.org/10.1038/s41589-019-0371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0371-x
This article is cited by
-
Gut microbiota-induced CXCL1 elevation triggers early neuroinflammation in the substantia nigra of Parkinsonian mice
Acta Pharmacologica Sinica (2024)
-
Investigation of brain iron in anorexia nervosa, a quantitative susceptibility mapping study
Journal of Eating Disorders (2023)
-
Perturbed iron biology in the prefrontal cortex of people with schizophrenia
Molecular Psychiatry (2023)
-
Hypoxia-inducible factor upregulation by roxadustat attenuates drug reward by altering brain iron homoeostasis
Signal Transduction and Targeted Therapy (2023)
-
Gut microbiota bridges the iron homeostasis and host health
Science China Life Sciences (2023)