Abstract
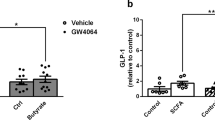
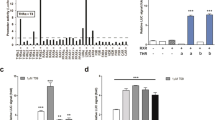
Differentiating actions of short chain fatty acids (SCFAs) at free fatty acid receptor 2 (FFA2) from other free fatty acid-responsive receptors and from non-receptor-mediated effects has been challenging. Using a novel chemogenetic and knock-in strategy, whereby an engineered variant of FFA2 (FFA2-DREADD) that is unresponsive to natural SCFAs but is instead activated by sorbic acid replaced the wild-type receptor, we determined that activation of FFA2 in differentiated adipocytes and colonic crypt enteroendocrine cells of mouse accounts fully for SCFA-regulated lipolysis and release of the incretin glucagon-like peptide-1 (GLP-1), respectively. In vivo studies confirmed the specific role of FFA2 in GLP-1 release and also demonstrated a direct role for FFA2 in accelerating gut transit. Thereby, we establish the general principle that such a chemogenetic knock-in strategy can successfully define novel G-protein-coupled receptor (GPCR) biology and provide both target validation and establish therapeutic potential of a ‘hard to target’ GPCR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data is available from the corresponding authors or is available through the University of Glasgow online data repository.
Code availability
Concentration-response curves analyzed using GraphPad Prism v.5.02.
References
Barratt, M. J., Lebrilla, C., Shapiro, H. Y. & Gordon, J. I. The gut microbiota, food science, and human nutrition: a timely marriage. Cell Host Microbe 22, 134–141 (2017).
McKenzie, C., Tan, J., Macia, L. & Mackay, C. R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 278, 277–295 (2017).
Stoddart, L. A., Smith, N. J. & Milligan, G. International union of pharmacology. LXXI. free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol. Rev. 60, 405–417 (2008).
Bolognini, D., Tobin, A. B., Milligan, G. & Moss, C. E. The pharmacology and function of receptors for short-chain fatty acids. Mol. Pharmacol. 89, 388–398 (2016).
Bindels, L. B., Dewulf, E. M. & Delzenne, N. M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 34, 226–232 (2013).
Milligan, G., Shimpukade, B., Ulven, T. & Hudson, B. D. Complex pharmacology of free fatty acid receptors. Chem. Rev. 117, 67–110 (2017).
Milligan, G., Bolognini, D. & Sergeev, E. Ligands at the free fatty acid receptors 2/3 (GPR43/GPR41). Handb. Exp. Pharmacol. 236, 17–32 (2017).
Sergeev, E. et al. A single extracellular amino acid in free fatty acid receptor 2 defines antagonist species selectivity and G protein selection bias. Sci. Rep. 7, 13741 (2017).
Namour, F. et al. Safety, pharmacokinetics and pharmacodynamics of GLPG0974, a potent and selective FFA2 antagonist, in healthy male subjects. Br. J. Clin. Pharmacol. 82, 139–148 (2016).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Urban, D. J. & Roth, B. L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
Smith, K. S., Bucci, D. J., Luikart, B. W. & Mahler, S. V. DREADDS: use and application in behavioral neuroscience. Behav. Neurosci. 130, 137–155 (2016).
Thiel, G., Kaufmann, A. & Rössler, O. G. G-protein-coupled designer receptors—new chemical-genetic tools for signal transduction research. Biol. Chem. 394, 1615–1622 (2013).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Bradley, S. J., Tobin, A. B. & Prihandoko, R. The use of chemogenetic approaches to study the physiological roles of muscarinic acetylcholine receptors in the central nervous system. Neuropharmacol. 136, 421–426 (2018).
Hudson, B. D. et al. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. FASEB J. 26, 4951–4965 (2012).
Ballesteros, J. A. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Meth. Neurosci. 255, 366–428 (2013).
Hudson, B. D. et al. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J. Biol. Chem. 288, 17296–17312 (2013).
Sergeev, E. et al. Non-equivalence of key positively charged residues of the free fatty acid 2 receptor in the recognition and function of agonist versus antagonist ligands. J. Biol. Chem. 291, 303–317 (2016).
Pizzonero, M. et al. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: from hit to clinic. J. Med. Chem. 57, 10044–10057 (2014).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Karamitri, A. et al. Type 2 diabetes-associated variants of the MT2 melatonin receptor affect distinct modes of signaling. Sci. Signal. 11, pii eaan6622 (2018).
Lee, S. U. et al. β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol. Pharm. Bull. 36, 1754–1759 (2013).
Ang, Z., Xiong, D., Wu, M. & Ding, J. L. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J. 32, 289–303 (2018).
Zaibi, M. S. et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 (2010).
Bolognini, D. et al. A novel allosteric activator of free fatty acid 2 receptor displays unique Gi-functional bias. J. Biol. Chem. 291, 18915–18931 (2016).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Nøhr, M. K. et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154, 3552–3564 (2013).
Li, Y., Kokrashvili, Z., Mosinger, B. & Margolskee, R. F. Gustducin couples fatty acid receptors to GLP-1 release in colon. Am. J. Physiol. Endocrinol. Metab. 304, E651–E660 (2013).
Engelstoft, M. S. et al. Research resource: a chromogranin A reporter for serotonin and histamine secreting enteroendocrine cells. Mol. Endocrinol. 29, 1658–1671 (2015).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 (2015).
Richardson, A., Delbridge, A. T., Brown, N. J., Rumsey, R. D. & Read, N. W. Short chain fatty acids in the terminal ileum accelerate stomach to caecum transit time in the rat. Gut 32, 266–269 (1991).
Tough, I. R., Forbes, S. & Cox, H. M. Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol. Motil. 30, e13454 (2018).
Hauser, A. S. et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Roth, B. L., Irwin, J. J. & Shoichet, B. K. Discovery of new GPCR ligands to illuminate new biology. Nat. Chem. Biol. 13, 1143–1151 (2017).
Milligan, G. G protein-coupled receptors not currently in the spotlight: free fatty acid receptor 2 and GPR35. Br. J. Pharmacol. 175, 2543–2553 (2018).
Viskaitis, P. et al. Modulation of SF1 neuron activity co-ordinately regulates both feeding behaviour and associated emotional states. Cell Rep. 21, 3559–3572 (2017).
Christiansen, C. B. et al. The impact of short chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G53–G65 (2018).
Ge, H. et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 (2008).
Lee, T. et al. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol. 74, 1599–1609 (2008).
Tang, C. & Offermanns, S. FFA2 and FFA3 in metabolic regulation. Handb. Exp. Pharmacol. 236, 205–220 (2017).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Acknowledgements
These studies were funded by Biotechnology and Biosciences Research Council grant nos. BB/L027887/1 (Milligan) and BB/L02781X/1 (Tobin) and a CIHR Foundation grant no. FDN-148431 (Bouvier). S.J.B. is funded through a University of Glasgow Lord Kelvin Adam Smith Fellowship and an MRC project grant (no. MR/P019366/1). We are also grateful to M. Hogue and V. Lukasheva for the design, construction and characterization of the GRK2-based biosensor. We acknowledge the BSU facilities at the Cancer Research UK Beatson Institute (C596/A17196) and the Biological Services at the University of Glasgow.
Author information
Authors and Affiliations
Contributions
G.M. and A.B.T. devised the program of work. G.M., A.B.T. and D.B. wrote the paper with assistance from all other authors. G.M., A.B.T., D.B. and N.B. designed the experiments. D.B. (genetic characterization, lipolysis, GLP-1 release, immunostaining, pharmacological characterization), A.J.B. (biosensor studies), E.S. (ligand binding studies), C.L.G. (biosensor studies), C.E.M. (GLP-1 release) and N.B. (GLP-1 release and gut transit) performed experiments. B.D.H. developed the hFFA2-DREADD receptor and performed initial characterization. M.B. directed the biosensor studies. S.J.B. and C.M. oversaw the animal-based studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Supplementary Table 1
Rights and permissions
About this article
Cite this article
Bolognini, D., Barki, N., Butcher, A.J. et al. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Nat Chem Biol 15, 489–498 (2019). https://doi.org/10.1038/s41589-019-0270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0270-1
This article is cited by
-
Chemogenetics for cell-type-specific modulation of signalling and neuronal activity
Nature Reviews Methods Primers (2023)
-
Revealing the tissue-level complexity of endogenous glucagon-like peptide-1 receptor expression and signaling
Nature Communications (2023)
-
From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators
Microbiome (2021)
-
Targeting lipid GPCRs to treat type 2 diabetes mellitus — progress and challenges
Nature Reviews Endocrinology (2021)
-
Oleate-induced aggregation of LC3 at the trans-Golgi network is linked to a protein trafficking blockade
Cell Death & Differentiation (2021)