Abstract
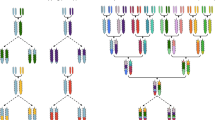
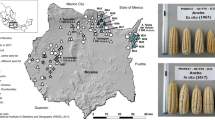
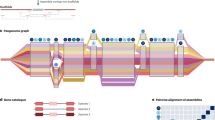
Genebanks have the long-term mission of preserving plant genetic resources as an agricultural legacy for future crop improvement. Operating procedures for seed storage and plant propagation have been in place for decades, but there is a lack of effective means for the discovery and transfer of beneficial alleles from landraces and wild relatives into modern varieties. Here, we review the prospects of using molecular passport data derived from genomic sequence information as a universal monitoring tool at the single-plant level within and between genebanks. Together with recent advances in breeding methodologies, the transformation of genebanks into bio-digital resource centers will facilitate the selection of useful genetic variation and its use in breeding programs, thus providing easy access to past crop diversity. We propose linking catalogs of natural genetic variation and enquiries into biological mechanisms of plant performance as a long-term joint research goal of genebanks, plant geneticists and breeders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Peres, S. Saving the gene pool for the future: seed banks as archives. Stud. Hist. Philos. Biol. Biomed. Sci. 55, 96–104 (2016).
Swarts, K. et al. Genomic estimation of complex traits reveals ancient maize adaptation to temperate North America. Science 357, 512–515 (2017).
Mascher, M. et al. Genomic analysis of 6,000-year-old cultivated grain illuminates the domestication history of barley. Nat. Genet. 48, 1089–1093 (2016).
Frankel, O. H. Genetic conservation: our evolutionary responsibility. Genetics 78, 53–65 (1974).
Hedden, P. The genes of the Green Revolution. Trends Genet. 19, 5–9 (2003).
Jørgensen, I. H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 63, 141–152 (1992).
Pistorius, R. Scientists, Plants and Politics: a History of the Plant Genetic Resources Movement (Bioversity International, 1997).
The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture (FAO, 2010).
Romay, M. C. et al. Comprehensive genotyping of the USA national maize inbred seed bank. Genome Biol. 14, R55 (2013).
Milner, S. G. et al. Genebank genomics highlights the diversity of a global barley collection. Nat. Genet. 51, 319–326 (2019).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Van Treuren, R., Van Soest, L. & Van Hintum, T. J. Marker-assisted rationalisation of genetic resource collections: a case study in flax using AFLPs. Theor. Appl. Genet. 103, 144–152 (2001).
Phippen, W. B., Kresovich, S., Candelas, F. G. & McFerson, J. R. Molecular characterization can quantify and partition variation among genebank holdings: a case study with phenotypically similar accessions of Brassica oleracea var. capitata L. (cabbage) ‘Golden Acre’. Theor. Appl. Genet. 94, 227–234 (1997).
van Hintum, T. J. L. & Visser, D. L. Duplication within and between germplasm collections. Genet. Resour. Crop Evol. 42, 135–145 (1995).
Gruber, K. Agrobiodiversity: the living library. Nature 544, S8–S10 (2017).
Parzies, H. K., Spoor, W. & Ennos, R. A. Genetic diversity of barley landrace accessions (Hordeum vulgare ssp. vulgare) conserved for different lengths of time in ex situ gene banks. Heredity 84, 476–486 (2000).
Weiß, C. L. et al. Temporal patterns of damage and decay kinetics of DNA retrieved from plant herbarium specimens. R. Soc. Open Sci. 3, 160239 (2016).
Alamerew, S., Chebotar, S., Huang, X., Röder, M. & Börner, A. Genetic diversity in Ethiopian hexaploid and tetraploid wheat germplasm assessed by microsatellite markers. Genet. Resour. Crop Evol. 51, 559–567 (2004).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Abbeloos, R. et al. BrAPI: an application programming interface for plant breeding applications. Bioinformatics https://doi.org/10.1093/bioinformatics/btz190 (2019).
Ćwiek-Kupczyńska, H. et al. Measures for interoperability of phenotypic data: minimum information requirements and formatting. Plant Methods 12, 44 (2016).
Marden, E. International agreements may impact genomic technologies. Nat. Plants 4, 2–4 (2018).
Crnokrak, P. & Barrett, S. C. Perspective: purging the genetic load: a review of the experimental evidence. Evolution 56, 2347–2358 (2002).
Romero Navarro, J. A. et al. A study of allelic diversity underlying flowering-time adaptation in maize landraces. Nat. Genet. 49, 476–480 (2017).
Böhm, J., Schipprack, W., Utz, H. F. & Melchinger, A. E. Tapping the genetic diversity of landraces in allogamous crops with doubled haploid lines: a case study from European flint maize. Theor. Appl. Genet. 130, 861–873 (2017).
Nelson, R., Wiesner-Hanks, T., Wisser, R. & Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 19, 21–33 (2018).
Corwin, J. A., Subedy, A., Eshbaugh, R. & Kliebenstein, D. J. Expansive phenotypic landscape of Botrytis cinerea shows differential contribution of genetic diversity and plasticity. Mol. Plant Microbe Interact. 29, 287–298 (2016).
Yano, K. et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 48, 927–934 (2016).
Bhullar, N. K., Street, K., Mackay, M., Yahiaoui, N. & Keller, B. Unlocking wheat genetic resources for the molecular identification of previously undescribed functional alleles at the Pm3 resistance locus. Proc. Natl. Acad. Sci. USA 106, 9519–9524 (2009).
Shan, Q., Wang, Y., Li, J. & Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 9, 2395–2410 (2014).
Nelson, P. T. & Goodman, M. M. Evaluation of elite exotic maize inbreds for use in temperate breeding. Crop Sci. 48, 85–92 (2008).
Longin, C. F. H. & Reif, J. C. Redesigning the exploitation of wheat genetic resources. Trends Plant Sci. 19, 631–636 (2014).
Yu, X. et al. Genomic prediction contributing to a promising global strategy to turbocharge gene banks. Nat. Plants 2, 16150 (2016).
Buckler, E. S. et al. The genetic architecture of maize flowering time. Science 325, 714–718 (2009).
Lorenz, A., Smith, K. & Jannink, J.-L. Potential and optimization of genomic selection for Fusarium head blight resistance in six-row barley. Crop Sci. 52, 1609–1621 (2012).
Akdemir, D., Sanchez, J. I. & Jannink, J.-L. Optimization of genomic selection training populations with a genetic algorithm. Genet. Sel. Evol. 47, 38 (2015).
Watson, A. et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 4, 23–29 (2018).
Gaynor, R. C. et al. A two-part strategy for using genomic selection to develop inbred lines. Crop Sci. 57, 2372–2386 (2017).
Zhao, Y. et al. Genome-based establishment of a high-yielding heterotic pattern for hybrid wheat breeding. Proc. Natl. Acad. Sci. USA 112, 15624–15629 (2015).
Wulff, B. B. H. & Dhugga, K. S. Wheat-the cereal abandoned by GM. Science 361, 451–452 (2018).
Zsögön, A. et al. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36, 1211–1216 (2018).
Li, T. et al. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36, 1160–116 (2018).
Lemmon, Z. H. et al. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 4, 766–770 (2018).
Blancke, S., Grunewald, W. & De Jaeger, G. De-problematizing ‘GMOs’: suggestions for communicating about genetic engineering. Trends Biotechnol. 35, 185–186 (2017).
Alberch, P. From genes to phenotype: dynamical systems and evolvability. Genetica 84, 5–11 (1991).
Davidson, E.H. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution (Elsevier, 2010).
Bernardo, R. & Yu, J. Prospects for genomewide selection for quantitative traits in maize. Crop Sci. 47, 1082–1090 (2007).
Lemmon, Z. H. et al. The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Res. 26, 1676–1686 (2016).
Kloosterman, B. et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 495, 246–250 (2013).
Simmonds, N. Variability in crop plants, its use and conservation. Biol. Rev. Camb. Philos. Soc. 37, 422–465 (1962).
Strigens, A., Schipprack, W., Reif, J. C. & Melchinger, A. E. Unlocking the genetic diversity of maize landraces with doubled haploids opens new avenues for breeding. PLoS One 8, e57234 (2013).
Milner, S. G. et al. Genebank genomics highlights the diversity of a global barley collection. Nat. Genet. 51, 319–326 (2019).
von Bothmer, R., Sato, K., Komatsuda, T., Yasuda, S. & Fischbeck, G. The domestication of cultivated barley. in Diversity in Barley (Hordeum vulgare) 9–27 (Elsevier, 2003).
Oppermann, M., Weise, S., Dittmann, C. & Knüpffer, H. GBIS: the information system of the German Genebank. Database (Oxf.) 2015, bav021 (2015).
Acknowledgements
This work was supported by grants from the German Federal Ministry of Research and Education (BMBF) to N.S., J.C.R., U.S. and M.M. (FKZ 031B0190; ‘SHAPE’; FKZ 031B0184 ‘Genebank 2.0’; FKZ 031A536A ‘de.NBI’) and a grant from the Leibniz Association to N.S., A.G., U.S., J.C.R. and M.M. (SAW-2015-IPK-1 ‘BRIDGE’). We thank M. Oppermann for tracing the origin of the Ethiopian accessions.
Author information
Authors and Affiliations
Contributions
The concept of this work was developed in discussions among N.S., A.G., J.C.R., U.S., M.S. and M.M. M.M. and J.C.R. wrote the paper, and all co-authors provided input. M.S. designed figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mascher, M., Schreiber, M., Scholz, U. et al. Genebank genomics bridges the gap between the conservation of crop diversity and plant breeding. Nat Genet 51, 1076–1081 (2019). https://doi.org/10.1038/s41588-019-0443-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-019-0443-6
This article is cited by
-
Host plants directly determine the α diversity of rhizosphere arbuscular mycorrhizal fungal communities in the National Tropical Fruit Tree Field Genebank
Chemical and Biological Technologies in Agriculture (2024)
-
Plant pangenomes for crop improvement, biodiversity and evolution
Nature Reviews Genetics (2024)
-
Genomic prediction for agronomic traits in a diverse Flax (Linum usitatissimum L.) germplasm collection
Scientific Reports (2024)
-
Are cereal grasses a single genetic system?
Nature Plants (2024)
-
A diverse panel of 755 bread wheat accessions harbors untapped genetic diversity in landraces and reveals novel genetic regions conferring powdery mildew resistance
Theoretical and Applied Genetics (2024)