Abstract
TET enzymes oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), which can lead to DNA demethylation. However, direct connections between TET-mediated DNA demethylation and transcriptional output are difficult to establish owing to challenges in distinguishing global versus locus-specific effects. Here we show that TET1, TET2 and TET3 triple-knockout (TKO) human embryonic stem cells (hESCs) exhibit prominent bivalent promoter hypermethylation without an overall corresponding decrease in gene expression in the undifferentiated state. Focusing on the bivalent PAX6 locus, we find that increased DNMT3B binding is associated with promoter hypermethylation, which precipitates a neural differentiation defect and failure of PAX6 induction during differentiation. dCas9-mediated locus-specific demethylation and global inactivation of DNMT3B in TKO hESCs partially reverses the hypermethylation at the PAX6 promoter and improves differentiation to neuroectoderm. Taking these findings together with further genome-wide methylation and TET1 and DNMT3B ChIP–seq analyses, we conclude that TET proteins safeguard bivalent promoters from de novo methylation to ensure robust lineage-specific transcription upon differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
18 December 2017
The version of the Supplementary Text and Figures file initially posted was missing Supplementary Tables 1–6 and the Supplementary Note and used incorrect versions of the supplementary figures.
18 December 2017
In the version of this article initially published, in the Methods, the Gene Expression Omnibus accession code for H3K36me3 ChIP–seq data was incorrectly given as GSM1003585 instead of GSM733725. The error has been corrected in the HTML, PDF and print versions of the article.
References
Ziller, M. J. et al. Charting a dynamic DNA methylation landscape of the human genome. Nature 500, 477–481 (2013).
Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005).
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Smith, Z. D. & Meissner, A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 14, 204–220 (2013).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010).
Guo, J. U., Su, Y., Zhong, C., Ming, G. L. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 (2011).
Dawlaty, M. M. et al. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev. Cell 29, 102–111 (2014).
Dai, H. Q. et al. TET-mediated DNA demethylation controls gastrulation by regulating Lefty–Nodal signalling. Nature 538, 528–532 (2016).
Lu, F., Liu, Y., Jiang, L., Yamaguchi, S. & Zhang, Y. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 28, 2103–2119 (2014).
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 (Suppl.), 245–254 (2003).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Rasmussen, K. D. & Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 30, 733–750 (2016).
Bogdanović, O. et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 48, 417–426 (2016).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Fouse, S. D. et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2, 160–169 (2008).
Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462, 315–322 (2009).
Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–770 (2008).
von Meyenn, F. et al. Comparative principles of DNA methylation reprogramming during human and mouse in vitro primordial germ cell specification. Dev. Cell 39, 104–115 (2016).
González, F. et al. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 15, 215–226 (2014).
Song, C. X. et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 29, 68–72 (2011).
Gu, H. et al. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat. Protoc. 6, 468–481 (2011).
Zhou, V. W., Goren, A. & Bernstein, B. E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 12, 7–18 (2011).
Zhang, X. et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90–100 (2010).
Li, X. J. et al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 23, 215–221 (2005).
Pankratz, M. T. et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511–1520 (2007).
Anderson, T. R., Hedlund, E. & Carpenter, E. M. Differential Pax6 promoter activity and transcript expression during forebrain development. Mech. Dev. 114, 171–175 (2002).
Kammandel, B. et al. Distinct cis-essential modules direct the time–space pattern of the Pax6 gene activity. Dev. Biol. 205, 79–97 (1999).
Plaza, S., Saule, S. & Dozier, C. High conservation of cis-regulatory elements between quail and human for the Pax-6 gene. Dev. Genes Evol. 209, 165–173 (1999).
Xu, Z. P. & Saunders, G. F. PAX6 intronic sequence targets expression to the spinal cord. Dev. Genet. 23, 259–263 (1998).
Zheng, J. B., Zhou, Y. H., Maity, T., Liao, W. S. & Saunders, G. F. Activation of the human PAX6 gene through the exon 1 enhancer by transcription factors SEF and Sp1. Nucleic Acids Res. 29, 4070–4078 (2001).
Stroud, H. et al. Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 12, R54 (2011).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Xu, X. et al. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2, 16009 (2016).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247 (2016).
Choudhury, S. R., Cui, Y., Lubecka, K., Stefanska, B. & Irudayaraj, J. CRISPR–dCas9 mediated TET1 targeting for selective DNA demethylation at BRCA1 promoter. Oncotarget 7, 46545–46556 (2016).
Morita, S. et al. Targeted DNA demethylation in vivo using dCas9–peptide repeat and scFv–TET1 catalytic domain fusions. Nat. Biotechnol. 34, 1060–1065 (2016).
Baubec, T. et al. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature 520, 243–247 (2015).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Pan, G. et al. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1, 299–312 (2007).
Guo, H. et al. The DNA methylation landscape of human early embryos. Nature 511, 606–610 (2014).
Ooi, S. K. et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448, 714–717 (2007).
Xu, Y. et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol. Cell 42, 451–464 (2011).
Neri, F. et al. Dnmt3L antagonizes DNA methylation at bivalent promoters and favors DNA methylation at gene bodies in ESCs. Cell 155, 121–134 (2013).
Liao, J. et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet. 47, 469–478 (2015).
Di Ruscio, A. et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503, 371–376 (2013).
Chen, T., Ueda, Y., Dodge, J. E., Wang, Z. & Li, E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 23, 5594–5605 (2003).
Liang, G. et al. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol. Cell. Biol. 22, 480–491 (2002).
Zhang, X. et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat. Genet. 48, 1014–1023 (2016).
Rinaldi, L. et al. Dnmt3a and Dnmt3b associate with enhancers to regulate human epidermal stem cell homeostasis. Cell Stem Cell 19, 491–501 (2016).
Pulecio, J., Verma, N., Meija-Ramirez, E., Huangfu, D. & Raya, A. CRISPR/Cas9-based engineering of the epigenome. Cell Stem Cell 21, 431–447 (2017).
Zhu, Z., González, F. & Huangfu, D. The iCRISPR platform for rapid genome editing in human pluripotent stem cells. Methods Enzymol. 546, 215–250 (2014).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572 (2011).
Akalin, A. et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet. 8, e1002781 (2012).
Pan, H. et al. Epigenomic evolution in diffuse large B-cell lymphomas. Nat. Commun. 6, 6921 (2015).
Giannopoulou, E. G. & Elemento, O. An integrated ChIP-seq analysis platform with customizable workflows. BMC Bioinformatics 12, 277 (2011).
Williams, K. et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348 (2011).
Ehrich, M. et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc. Natl. Acad. Sci. USA 102, 15785–15790 (2005).
Acknowledgements
We thank the WCMC Epigenomics core for ERRBS and 5mC MassARRAY analysis and the MSKCC Integrated Genomics core for performing RNA-seq and WGBS. We also thank L. Studer and J. Tchieu for advice on neuroectoderm differentiation, E. Apostolou and M. Donohoe for comments on the manuscript, and M. Goll and members of the Huangfu laboratory for insightful discussions and critical reading of the manuscript. This study was funded in part by the Tri-Institutional Stem Cell Initiative (2016-032), New York State Stem Cell Science (NYSTEM C029156) and an MSKCC Cancer Center Support grant (P30 CA008748). N.V. is supported by the Weill Graduate School of Medical Sciences at the Cornell University/The Rockefeller University/Sloan Kettering Institute Tri-Institutional MD–PhD Program.
Author information
Authors and Affiliations
Contributions
N.V. and D.H. devised experiments and interpreted results. N.V. performed most experiments and collected data. H.P. and O.E. performed computational analysis on WGBS, ERRBS, RNA-seq, ChIP–seq and 5hmC profiling. L.C.D. and C.H. performed 5hmC profiling, sequencing and analysis, and mass spectrometry. A.K. generated libraries for WGBS. A.S., Q.V.L., B.P.-W., V.T., F.G., E.P.P. and C.-J.C. assisted with additional experiments. N.V. and D.H. wrote the manuscript; all other authors provided editorial advice.
Corresponding authors
Ethics declarations
Competing interests
5hmC-Seal has been licensed to Active Motif and Epican by the University of Chicago. This statement is relevant to C.H.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
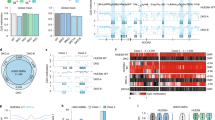
Supplementary Figure 1 5hmC and 5mC analysis of TKO hESCs.
a, TET expression in WT hESCs by RNA-seq; n = 2 independent experiments. Data are presented as means ± s.d. b, Analysis of 5hmC levels in WT and knockout hESCs by 5hmC dot blot. Human fibroblasts (Fib CTRL) are used as a negative control. c, Analysis of 5hmC (left) and 5mC (right) levels in WT and TKO MEL-1 hESCs by mass spectrometry. For WT cells, two different lines were used for mass spectrometry measurements; for TKO cells, two different passages of the same line were used for mass spectrometry measurements. Human fibroblasts were used as a negative control for mass spectrometry analysis of 5hmC. Data are presented as means ± s.d. Black lines indicate comparisons to WT. Statistical analysis was performed by one-way ANOVA: *P < 0.05. d, FACS analysis for pluripotency-associated cell-surface markers TRA-1-60 and TRA-1-81 in WT and TKO hESCs; the fluorescence intensity (left) and percentage positive cells (right) are shown; n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided). e, Methylation levels at HOXA7 and HOXA9 promoters in WT and TKO hESCs by WGBS; n = 1 independent experiment. The x-axis numbers correspond to the positions of CpG sites in the hg19 genome assembly
Supplementary Figure 2 Hypermethylation of bivalent promoters after TET inactivation.
a, GO analysis for genes associated with hypermethylated bivalent promoters (n = 1,693), genes associated with hypermethylated poised enhancers (n = 3,570) and genes associated with hypermethylated active enhancers (n = 3,805). FDR < 0.01 was set as a cutoff for bivalent promoters and poised enhancers, and FDR < 0.05 was set as a cutoff for active enhancers. Developmental categories are in red. b, Fraction of genomic regions showing a >5% increase in 5mC (Hyper) or a >5% decrease in 5mC (Hypo) for TKO versus WT hESCs by ERRBS of HUES8 and MEL-1 WT and TKO hESCs; n = 2 independent experiments. c, DNA methylation change between TKO and WT MEL-1 hESCs by ERRBS at different promoter types. Box-and-whisker plots were generated using the methylation change at individual promoters. The error bars show 10% and 90% confidence intervals. The lower and upper limits of the box represent the first and third quartile, respectively, and the bar at the center of the plot box indicates the median. The promoters are divided into four groups based on histone modification patterns. The details of promoter definitions can be found in the Methods; n = 2 independent experiments. Statistical analysis was performed by one-way ANOVA: **P < 0.01, ****P < 0.0001. d, Representation of bivalent promoters among promoters that show different degrees of methylation change between TKO and WT MEL-1 hESCs by ERRBS; n indicates the total number of promoters in each DNA methylation change group, with n = 2 independent experiments. e, Correlation between the methylation level at bivalent promoters in HUES8 WT, MEL-1 WT, HUES8 TKO and MEL-1 TKO hESCs by ERRBS; n = 2 independent experiments. f, Overlap between all hyper-DMR-associated bivalent promoters in mESCs11 and their human counterparts in hESCs (by ERRBS, n = 2 independent experiments). The odds ratio and P value are given (Fisher’s exact test)
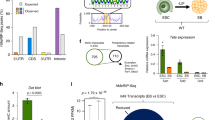
Supplementary Figure 3 TKO hESCs show few transcriptional changes at hypermethylated bivalent promoters.
a, Volcano plot of RNA-seq data illustrating transcriptional changes in TKO hESCs as compared to WT hESCs. Genes with a count difference greater than twofold and with adjusted P < 0.05 are shown in color (blue, upregulated; red, downregulated); n = 2 independent experiments. b, Top, expression changes for genes with hyper-DMR-associated promoters. Significance tests compared hyper-DMR-associated active, initiated, bivalent and silent promoters to all promoters together. Bottom, expression changes for genes with hyper-DMR-associated active enhancers and for genes with hyper-DMR-associated poised enhancers. Significance tests compared hyper-DMR-associated enhancers to all enhancers together. The details of each of these classifications are provided in the Methods. Box-and-whisker plots were generated using the expression changes for genes associated with individual hypermethylated promoters or enhancers. The error bars show 10% and 90% confidence intervals. The lower and upper limits of the box represent the first and third quartile, respectively, and the bar at the center of the plot box indicates the median. n = 2 independent experiments. Statistical analysis was performed by one-way ANOVA: ****P < 0.0001. c, Heat map of MassARRAY analysis of 5mC at different active promoters (GAPDH, OCT4, NANOG and SOX2) and bivalent promoters (FOXA2, GATA2, SOX10, SOX17 and PAX6). The location of each row of CpGs with respect to the TSS is shown to the left of each heat map. The color key for percent methylation is shown below the heat maps. For each cell line, three independent experiments are shown as three columns; n = 3 independent experiments. Statistical analysis was performed by Student’s t test (two-sided): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. d, Expression analysis by RNA-seq for particular genes; n = 2 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided): *P < 0.05, **P < 0.01, ***P < 0.001
Supplementary Figure 4 Hyper-DMRs in TKO hESCs overlap with 5hmC and TET1 peaks in WT hESCs.
a, Diagram of the PAX6 locus and the associated regulatory regions. Boxes on the horizontal line represent exons. Gray boxes represent the coding sequence. The two promoters that produce full-length protein (P0 and P1) are shown by arrows. The five annotated enhancers are depicted as yellow boxes. b, Heat map of MassARRAY analysis of 5mC at the regulatory regions of the PAX6 locus; n = 1 independent experiment. The color key for percent methylation is shown below the heat maps. c, Heat map of MassARRAY analysis of 5mC at the PAX6 P0 promoter in WT and TKO MEL-1 hESCs. The location of each row of CpGs with respect to the TSS is shown to the left of the heat map. The color key for percent methylation is shown to the right of the heat map. For each cell line, three biological replicates are shown as three columns; n = 3 independent experiments. Statistical analysis was performed by Student’s t test (two-sided): ***P < 0.001. d, Overlap of 5hmC and TET1 peaks found at promoters. e, Percentage of TET1 peaks overlapping 5hmC peaks in WT hESCs as compared to randomly generated 5hmC peaks of equal number and height. f, Analysis of TET1 (left) and 5hmC (right) peaks at active, initiated, bivalent and silent promoters in WT hESCs. The height above the x axis reflects the normalized tag count. g, Methylation change for active, initiated and silent promoters with 5hmC peaks in WT hESCs as compared to active, initiated and silent promoters without 5hmC peaks in WT hESCs. h, Overlap of hyper-DMRs (TKO versus WT) that occur at bivalent promoters with 5hmC (left) and TET1 (right) peaks at bivalent promoters in WT hESCs
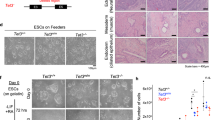
Supplementary Figure 5 HUES8 and MEL-1 TKO hESCs show impaired differentiation into the neuroectoderm lineage.
a, Expression of epiblast (OTX2), neuroectoderm (OTX2, PAX6, SOX1) and neural crest (SOX10) markers in WT cells during neuroectoderm differentiation; n = 3 independent experiments. Data are presented as means ± s.d. b, Expression of neuroectoderm markers (FOXG1, LHX2) at day 10 of neuroectoderm differentiation in WT and TKO cells; n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided): **P < 0.01. c, Immunofluorescence of PAX6, SOX1 and OCT4 at day 10 of neuroectoderm differentiation in WT and TKO MEL-1 cells. Scale bar, 100 μm. d, Expression of neuroectoderm (PAX6, SOX1) markers during neuroectoderm differentiation of WT and TKO MEL-1 lines; n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided): *P < 0.05, **P < 0.01, ***P < 0.001
Supplementary Figure 6 Neuroectoderm differentiation is sensitive to TET gene dosage.
a, Representative FACS plots of PAX6-positive cells at day 10 of neuroectoderm differentiation for TET-knockout mutants. b, Immunofluorescence of PAX6, SOX1 and OCT4 at the endpoint of differentiation (day 10) of TET-knockout mutants. Scale bar, 100 μm. c, Expression of neuroectoderm markers (PAX6, SOX1) at day 10 of neuroectoderm differentiation; n = 3 independent experiments. Data are presented as means ± s.d. For significance tests, all comparisons are to WT. Statistical analysis: was performed by one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Supplementary Figure 7 Partial rescue of TKO phenotypes through PAX6 overexpression and targeted demethylation of the PAX6 P0 promoter.
a, Expression of neuroectoderm (endogenous PAX6:PAX6 (Endo), total PAX6:PAX6 (All), and FOXG1), neural crest (SOX10) and pluripotency (NANOG) markers at day 10 of neuroectoderm differentiation of WT cells, TKO cells without doxycycline treatment (TKO) and TKO cells treated with doxycycline during neuroectoderm differentiation (TKO + PAX6). n = 3 independent experiments. Data are presented as means ± s.d. For significance tests, black lines indicate comparison with WT. Statistical analysis was performed by one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. b, Diagram of the dCas9–TET1 catalytic domain fusion protein (dCas9-TET1CD) and the dCas9–TET1 catalytic domain fusion protein in which the TET1 catalytic domain has been mutated (dCas9-TET1CD/Mut). c, Diagram of the gRNAs (blue arrows) designed to target the PAX6 P0 promoter in the region surrounding the 5hmC peak found in WT hESCs. The arrowhead indicates the 5′ end of the targeting gRNA. Regions previously analyzed for TET1 binding by ChIP–qPCR are enclosed by black rectangles. d, Heat map of MassARRAY analysis of 5mC in nontransfected TKO dCas9-TET1CD hESCs (NT) and TKO dCas9-TET1CD hESCs transiently transfected with gRNAs targeting the PAX6 P0 promoter (Cr6, Cr7, Cr9). The location of each row of CpGs with respect to the TSS is shown to the left of the heat map. The color key for percent methylation is shown to the right of the heat map. The graph on the bottom shows quantification of methylation in the region depicted in the heat map; n = 2 independent experiments. Data are presented as means ± s.d. For significance tests, all comparisons are to the nontransfected control (NT). Statistical analysis was performed by Student’s t test (two-sided): **P < 0.01, ***P < 0.001. e, Targeted demethylation at the bivalent promoters of PAX6 (left) and SOX10 (middle) and the enhancer of LEFTY2 (right). We used dCas9-TET1CD-targeted TKO hESCs expressing an HBB-targeting gRNA as a nontargeting (NT) control. Top, heat map of MassARRAY analysis of 5mC at the bivalent promoters of PAX6 and SOX10 and the enhancer of LEFTY2 after dCas9-TET1CD-mediated demethylation with either nontargeting or targeting gRNAs. – DOX indicates that the cell lines were not treated with doxycycline and thus do not express dCas9-TET1CD. The location of each row of CpGs with respect to the TSS is shown to the left of each heat map. The color key for percent methylation is shown to the right of the LEFTY2 heat maps. For each cell line and condition, three independent experiments are shown as three columns. Statistical analysis was performed by one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Bottom, qPCR analysis of targeted gene expression either in hESCs (blue) or after neuroectoderm differentiation (red); n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Supplementary Figure 8 DNMT3B deletion partially rescues bivalent promoter hypermethylation in TKO hESCs.
a, Expression of DNMT1, DNMT3A and DNMT3B in WT and TKO hESCs by RNA-seq analysis; n = 2 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided). b, ChIP–qPCR for DNMT1 (left) and DNMT3A (right) at the PAX6 locus in WT and TKO hESCs. Primers are the same ones used for Figs. 3g and 6a. n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided). c, Methylation change in QKO versus TKO lines at active, initiated, bivalent and silent promoters. Error bars show 10% and 90% confidence intervals. The lower and upper limits of the box represent the first and third quartile, respectively, and the bar at the center of the plot box indicates the median. n = 2 independent experiments. Statistical analysis was performed by one-way ANOVA: *P < 0.05, ****P < 0.0001. d, Percentage methylation in WT, TKO and QKO hESCs in a 10-kb region surrounding active, initiated, bivalent and silent promoters. e, Left, fraction of bivalent genes and total genes overlapping hyper-DMRs (HUES8 and MEL-1 TKO versus HUES8 and MEL-1 WT hESCs) and hypo-DMRs (HUES8 QKO versus HUES8 TKO hESCs). Hyper-DMRs and hypo-DMRs are from ERRBS datasets. Right, fraction of bivalent genes and total genes overlapping 5hmC and TET1 peaks in HUES8 WT hESCs, and DNMT3B peaks in HUES8 WT and TKO hESCs. f, ChIP–qPCR for H3K4me3 (top) and H3K27me3 (bottom) histone marks in HUES8 WT and TKO hESCs. For the P0 promoter, two primer pairs were used (ChIP qPCR: PAX6 P0 Promoter 1 and 2 in Supplementary Table 11). For the P1 promoter, one primer pair was used (ChIP qPCR: PAX6 P1 Promoter 1 in Supplementary Table 11). n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by Student’s t test (two-sided): *P < 0.05, **P < 0.01. g, DNMT1, DNMT3A and DNMT3B expression in HUES8 WT, TKO and QKO hESCs by RT–qPCR. n = 3 independent experiments. Data are presented as means ± s.d. Statistical analysis was performed by one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–6 and Supplementary Note
Supplementary Data 1
Hyper-DMRs for TKO as compared to WT HUES8 hESCs by WGBS. The number of differentially methylated CpGs per hyper-DMR is provided along with the methylation difference between WT and TKO HUES8 hESCs. Data from one run of WGBS are presented.
Supplementary Data 2
DNA methylation levels at bivalent promoters in WT and TKO HUES8 hESCs by WGBS. The average methylation at each bivalent promoter as well as the methylation difference (TKO – WT) and the fold change (TKO/WT) is provided. The definitions used for promoters are provided in the Methods. Data from one run of WGBS are presented.
Supplementary Data 3
Hyper-DMRs for TKO as compared to WT HUES8 hESCs by ERRBS. The number of differentially methylated CpGs per hyper-DMR is provided along with the methylation difference between WT and TKO HUES8 hESCs. The combined analysis of two replicates of ERRBS is presented.
Supplementary Data 4
Hyper-DMRs for TKO as compared to WT MEL-1 hESCs by ERRBS. The number of differentially methylated CpGs per hyper-DMR is provided along with the methylation difference between WT and TKO MEL-1 hESCs. The combined analysis of two replicates of ERRBS is presented.
Supplementary Data 5
DNA methylation levels at bivalent promoters in WT and TKO HUES8 hESCs by ERRBS. The average methylation at each bivalent promoter as well as the methylation difference (TKO – WT) and the fold change (TKO/WT) is provided. The definitions used for promoters are provided in the Methods. The combined analysis of two replicates of ERRBS is presented.
Supplementary Data 6
DNA methylation levels at bivalent promoters in WT and TKO MEL-1 hESCs by ERRBS. The average methylation at each bivalent promoter as well as the methylation difference (TKO – WT) and the fold change (TKO/WT) is provided. The definitions used for promoters are provided in the Methods. The combined analysis of two replicates of ERRBS is presented.
Supplementary Data 7
Differentially expressed genes between TKO and WT HUES8 hESCs. The log2-transformed fold change (TKO/WT), P value and adjusted P value (Benjamini–Hochberg correction) are provided. The combined analysis of three replicates of RNA-seq is presented.
Supplementary Data 8
DNA methylation levels at bivalent promoters in WT, TKO and QKO HUES8 hESCs and in WT and TKO MEL-1 hESCs by ERRBS. The average methylation at each bivalent promoter is provided. The individual replicates of the ERRBS are provided.
Rights and permissions
About this article
Cite this article
Verma, N., Pan, H., Doré, L.C. et al. TET proteins safeguard bivalent promoters from de novo methylation in human embryonic stem cells. Nat Genet 50, 83–95 (2018). https://doi.org/10.1038/s41588-017-0002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-017-0002-y
This article is cited by
-
Regulation, functions and transmission of bivalent chromatin during mammalian development
Nature Reviews Molecular Cell Biology (2023)
-
Crosstalk between RNA m6A and DNA methylation regulates transposable element chromatin activation and cell fate in human pluripotent stem cells
Nature Genetics (2023)
-
TET (Ten-eleven translocation) family proteins: structure, biological functions and applications
Signal Transduction and Targeted Therapy (2023)
-
Blockade of IL-6 signaling alleviates atherosclerosis in Tet2-deficient clonal hematopoiesis
Nature Cardiovascular Research (2023)
-
Dynamic antagonism between key repressive pathways maintains the placental epigenome
Nature Cell Biology (2023)