Abstract
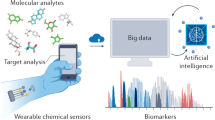
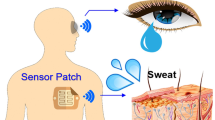
Wearable biosensors are garnering substantial interest due to their potential to provide continuous, real-time physiological information via dynamic, noninvasive measurements of biochemical markers in biofluids, such as sweat, tears, saliva and interstitial fluid. Recent developments have focused on electrochemical and optical biosensors, together with advances in the noninvasive monitoring of biomarkers including metabolites, bacteria and hormones. A combination of multiplexed biosensing, microfluidic sampling and transport systems have been integrated, miniaturized and combined with flexible materials for improved wearability and ease of operation. Although wearable biosensors hold promise, a better understanding of the correlations between analyte concentrations in the blood and noninvasive biofluids is needed to improve reliability. An expanded set of on-body bioaffinity assays and more sensing strategies are needed to make more biomarkers accessible to monitoring. Large-cohort validation studies of wearable biosensor performance will be needed to underpin clinical acceptance. Accurate and reliable real-time sensing of physiological information using wearable biosensor technologies would have a broad impact on our daily lives.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bandodkar, A. J., Jeerapan, I. & Wang, J. Wearable chemical sensors: present challenges and future prospects. ACS Sens. 1, 464–482 (2016).
Heikenfeld, J. et al. Wearable sensors: modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018).
Matzeu, G., Florea, L. & Diamond, D. Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B Chem. 211, 403–418 (2015).
Liu, Y., Pharr, M. & Salvatore, G. A. Lab-on-skin: a review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 11, 9614–9635 (2017).
Amjadi, M., Kyung, K. U., Park, I. & Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: a review. Adv. Funct. Mater. 26, 1678–1698 (2016).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Clark, L. C. Jr. & Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. NY Acad. Sci. 102, 29–45 (1962).
Abdellatif, M. S., Suleiman, A. A. & Guilbault, G. G. Enzyme-based fiber optic sensor for glucose determination. Anal. Lett. 21, 943–951 (1988).
Arnold, M. A. Enzyme-based fiber optic sensor. Anal. Chem. 57, 565–566 (1985).
Seitz, W. R. Chemical sensors based on fiber optics. Anal. Chem. 56, 16A–34A (1984).
Ward, M. D. & Buttry, D. A. In situ interfacial mass detection with piezoelectric transducers. Science 249, 1000–1007 (1990).
Muramatsu, H., Dicks, J. M., Tamiya, E. & Karube, I. Piezoelectric crystal biosensor modified with protein A for determination of immunoglobulins. Anal. Chem. 59, 2760–2763 (1987).
Janshoff, A., Galla, H. J. & Steinem, C. Piezoelectric mass-sensing devices as biosensors—an alternative to optical biosensors? Angew. Chem. Int. Ed. Engl. 39, 4004–4032 (2000).
Ngeh-Ngwainbi, J., Suleiman, A. A. & Guilbault, G. G. Piezoelectric crystal biosensors. Biosens. Bioelectron. 5, 13–26 (1990).
Hilditch, P. I. & Green, M. J. Disposable electrochemical biosensors. Analyst 116, 1217–1220 (1991).
Frew, J. E. & Hill, H. A. O. Electrochemical biosensors. Anal. Chem. 59, 933A–944A (1987).
Bindra, D. S. et al. Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal. Chem. 63, 1692–1696 (1991).
Wilson, G. S. & Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 20, 2388–2403 (2005).
Rodbard, D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol. Ther. 18 (Suppl. 2), S3–S13 (2016).
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 23, 1294–1301 (2005).
Pokorski, J. K., Nam, J. M., Vega, R. A., Mirkin, C. A. & Appella, D. H. Cyclopentane-modified PNA improves the sensitivity of nanoparticle-based scanometric DNA detection. Chem. Commun. (Camb.) 0, 2101–2103 (2005).
Thorp, H. H. Cutting out the middleman: DNA biosensors based on electrochemical oxidation. Trends Biotechnol. 16, 117–121 (1998).
Wang, J. & From, D. N. A. biosensors to gene chips. Nucleic Acids Res. 28, 3011–3016 (2000).
Odenthal, K. J. & Gooding, J. J. An introduction to electrochemical DNA biosensors. Analyst 132, 603–610 (2007).
Wang, J. Electrochemical glucose biosensors. Chem. Rev. 108, 814–825 (2008).
Johnson, B. N. & Mutharasan, R. Biosensing using dynamic-mode cantilever sensors: a review. Biosens. Bioelectron. 32, 1–18 (2012).
Newman, J. D. & Turner, A. P. Home blood glucose biosensors: a commercial perspective. Biosens. Bioelectron. 20, 2435–2453 (2005).
Mani, V., Chikkaveeraiah, B. V., Patel, V., Gutkind, J. S. & Rusling, J. F. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 3, 585–594 (2009).
Kim, J., Campbell, A. S. & Wang, J. Wearable non-invasive epidermal glucose sensors: a review. Talanta 177, 163–170 (2018).
Tierney, M. J., Tamada, J. A., Potts, R. O., Jovanovic, L. & Garg, S. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 16, 621–629 (2001).
Vashist, S. K. Continuous glucose monitoring systems: a review. Diagnostics (Basel) 3, 385–412 (2013).
Mannoor, M. S. et al. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 3, 763 (2012).
Senior, M. Novartis signs up for Google smart lens. Nat. Biotechnol. 32, 856 (2014).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Bandodkar, A. J. et al. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. 87, 394–398 (2015).
Martín, A. et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2, 1860–1868 (2017).
Sempionatto, J. R. et al. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 17, 1834–1842 (2017).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Jeerapan, I., Sempionatto, J. R., Pavinatto, A., You, J. M. & Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A Mater. Energy Sustain. 4, 18342–18353 (2016).
Munje, R. D., Muthukumar, S., Jagannath, B. & Prasad, S. A new paradigm in sweat based wearable diagnostics biosensors using room temperature ionic liquids (RTILs). Sci. Rep. 7, 1950 (2017).
Imani, S. et al. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Kim, J., Kumar, R., Bandodkar, A. J. & Wang, J. Advanced materials for printed wearable electrochemical devices: a review. Adv. Electron. Mater. 3, 1600260 (2017).
Bandodkar, A. J., Mohan, V., Lopez, C. S., Ramirez, J. & Wang, J. Self-healing inks for autonomous repair of printable electrochemical devices. Adv. Electron. Mater. 1, 1500289 (2015).
Wu, H., Gao, W. & Yin, Z. Materials, devices and systems of soft bioelectronics for precision therapy. Adv. Healthc. Mater. 6, 1700017 (2017).
Stoppa, M. & Chiolerio, A. Wearable electronics and smart textiles: a critical review. Sensors (Basel) 14, 11957–11992 (2014).
Bandodkar, A. J. & Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371 (2014).
Bandodkar, A. J., Jia, W. Z. & Wang, J. Tattoo-based wearable electrochemical devices: a review. Electroanalysis 27, 562–572 (2015).
Jin, H., Abu-Raya, Y. S. & Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 6, 1700024 (2017).
Piwek, L., Ellis, D. A., Andrews, S. & Joinson, A. The rise of consumer health wearables: promises and barriers. PLoS Med. 13, e1001953 (2016).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. https://doi.org/10.1039/c7cs00730b (2018).
Xue, X. Y. et al. Self-powered electronic-skin for detecting glucose level in body fluid basing on piezo-enzymatic-reaction coupling process. Nano Energy 26, 148–156 (2016).
Han, W. et al. A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays. ACS Appl. Mater. Interfaces 9, 29526–29537 (2017).
Morris, D. et al. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuators B Chem. 139, 231–236 (2009).
Windmiller, J. R. & Wang, J. Wearable electrochemical sensors and biosensors: a review. Electroanalysis 25, 29–46 (2013).
Jia, W. et al. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85, 6553–6560 (2013).
Gualandi, I. et al. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep. 6, 33637 (2016).
Caldara, M., Colleoni, C., Guido, E., Re, V. & Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 222, 213–220 (2016).
Choi, D. H., Kim, J. S., Cutting, G. R. & Searson, P. C. Wearable potentiometric chloride sweat sensor: the critical role of the salt bridge. Anal. Chem. 88, 12241–12247 (2016).
Liu, J., Liu, R. & Xu, K. Accuracy of noninvasive glucose sensing based on near-infrared spectroscopy. Appl. Spectrosc. 69, 1313–1318 (2015).
Ben Mohammadi, L. et al. In vivo evaluation of a chip based near infrared sensor for continuous glucose monitoring. Biosens. Bioelectron. 53, 99–104 (2014).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Thennadil, S. N. et al. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol. Ther. 3, 357–365 (2001).
Campbell, A. S., Kim, J. & Wang, J. Wearable electrochemical alcohol biosensors. Curr. Opin. Electrochem. 10, 126–135 (2018).
Fogh-Andersen, N., Altura, B. M., Altura, B. T. & Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 41, 1522–1525 (1995).
Venugopal, M. et al. Clinical evaluation of a novel interstitial fluid sensor system for remote continuous alcohol monitoring. IEEE Sens. J. 8, 71–80 (2008).
Venugopal, M., Arya, S. K., Chornokur, G. & Bhansali, S. A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sens. Actuators A Phys. 172, 154–160 (2011).
Bandodkar, A. J. et al. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 138, 123–128 (2013).
Bandodkar, A. J. et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 54, 603–609 (2014).
Guinovart, T., Bandodkar, A. J., Windmiller, J. R., Andrade, F. J. & Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 138, 7031–7038 (2013).
Kim, J. et al. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 51, 41–45 (2015).
Kim, J. et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 1, 1011–1019 (2016).
Wang, L. et al. Weaving sensing fibers into electrochemical fabric for real‐time health monitoring. Adv. Funct. Mater. 28, 1804456 (2018).
Moyer, J., Wilson, D., Finkelshtein, I., Wong, B. & Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14, 398–402 (2012).
Sakaguchi, K. et al. Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J. Diabetes Sci. Technol. 7, 678–688 (2013).
Lee, H. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, e1601314 (2017).
Curto, V. F. et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 171, 1327–1334 (2012).
Mu, X. et al. A paper-based skin patch for the diagnostic screening of cystic fibrosis. Chem. Commun. (Camb.) 51, 6365–6368 (2015).
Huang, X. et al. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 10, 3083–3090 (2014).
Oncescu, V., O’Dell, D. & Erickson, D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 13, 3232–3238 (2013).
Kim, S. B. et al. Super-absorbent polymer valves and colorimetric chemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small 14, e1703334 (2018).
Sekine, Y. et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 18, 2178–2186 (2018).
Kinnamon, D., Ghanta, R., Lin, K. C., Muthukumar, S. & Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 7, 13312 (2017).
Ciui, B. et al. Wearable wireless tyrosinase bandage and microneedle sensors: toward melanoma screening. Adv. Healthc. Mater. 7, e1701264 (2018).
Chen, Y. et al. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 3, e1701629 (2017).
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Gibson, L. E. & Cooke, R. E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23, 545–549 (1959).
Cole, D. E. C. & Boucher, M. J. Use of a new sample-collection device (Macroduct) in anion analysis of human sweat. Clin. Chem. 32, 1375–1378 (1986).
Matzeu, G., Fay, C., Vaillant, A., Coyle, S. & Diamond, D. A wearable device for monitoring sweat rates via image analysis. IEEE Trans. Biomed. Eng. 63, 1672–1680 (2016).
Tai, L. C. et al. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 30, e1707442 (2018).
Kim, J. et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. (Weinh.) 5, 1800880 (2018).
Farandos, N. M., Yetisen, A. K., Monteiro, M. J., Lowe, C. R. & Yun, S. H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 4, 792–810 (2015).
Baca, J. T., Finegold, D. N. & Asher, S. A. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul. Surf. 5, 280–293 (2007).
Thaysen, J. H. & Thorn, N. A. Excretion of urea, sodium, potassium and chloride in human tears. Am. J. Physiol. 178, 160–164 (1954).
Mitsubayashi, K. & Arakawa, T. Cavitas sensors: contact lens type sensors & mouthguard sensors. Electroanalysis 28, 1170–1187 (2016).
Pankratov, D., Gonzalez-Arribas, E., Blum, Z. & Shleev, S. Tear based bioelectronics. Electroanalysis 28, 1250–1266 (2016).
Sen, D. K. & Sarin, G. S. Tear glucose levels in normal people and in diabetic patients. Br. J. Ophthalmol. 64, 693–695 (1980).
Chu, M. X. et al. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 83, 960–965 (2011).
Chu, M. et al. Biomedical soft contact-lens sensor for in situ ocular biomonitoring of tear contents. Biomed. Microdevices 13, 603–611 (2011).
Mishima, S., Gasset, A., Klyce, S. D. Jr. & Baum, J. L. Determination of tear volume and tear flow. Invest. Ophthalmol. 5, 264–276 (1966).
Fullard, R. J. & Carney, L. G. Diurnal variation in human tear enzymes. Exp. Eye Res. 38, 15–26 (1984).
Yan, Q. et al. Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal. Chem. 83, 8341–8346 (2011).
Stuchell, R. N., Feldman, J. J., Farris, R. L. & Mandel, I. D. The effect of collection technique on tear composition. Invest. Ophthalmol. Vis. Sci. 25, 374–377 (1984).
Green-Church, K. B., Nichols, K. K., Kleinholz, N. M., Zhang, L. & Nichols, J. J. Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis. 14, 456–470 (2008).
Alexeev, V. L., Das, S., Finegold, D. N. & Asher, S. A. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 50, 2353–2360 (2004).
Ben-Moshe, M., Alexeev, V. L. & Asher, S. A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 78, 5149–5157 (2006).
Domschke, A., March, W. F., Kabilan, S. & Lowe, C. Initial clinical testing of a holographic non-invasive contact lens glucose sensor. Diabetes Technol. Ther. 8, 89–93 (2006).
Khalil, O. S. Noninvasive photonic-crystal material for sensing glucose in tears. Clin. Chem. 50, 2236–2237 (2004).
Cai, Z., Smith, N. L., Zhang, J. T. & Asher, S. A. Two-dimensional photonic crystal chemical and biomolecular sensors. Anal. Chem. 87, 5013–5025 (2015).
Elsherif, M., Hassan, M. U., Yetisen, A. K. & Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12, 5452–5462 (2018).
Sung, Y., Campa, F. & Shih, W. C. Open-source do-it-yourself multi-color fluorescence smartphone microscopy. Biomed. Opt. Express 8, 5075–5086 (2017).
Yao, H., Afanasiev, A., Lahdesmaki, I. & Parviz, B. A. A dual microscale glucose sensor on a contact lens, tested in conditions mimicking the eye. Proc. IEEE Micro. Electro. Mech. Syst. 2011, 25–28 (2011).
Yao, H. et al. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 22, 075007 (2012).
Liao, Y. T., Yao, H. F., Lingley, A., Parviz, B. & Otis, B. P. A. 3-µW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 47, 335–344 (2012).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Park, J. et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 4, eaap9841 (2018).
Kownacka, A. E. et al. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing biopolymer coating. Biomacromolecules 19, 4504–4511 (2018).
Zubareva, T. V. & Kiseleva, Z. M. Catecholamine content of the lacrimal fluid of healthy people and glaucoma patients. Ophthalmologica 175, 339–344 (1977).
Zhou, L. et al. In-depth analysis of the human tear proteome. J. Proteomics 75, 3877–3885 (2012).
Pfaffe, T., Cooper-White, J., Beyerlein, P., Kostner, K. & Punyadeera, C. Diagnostic potential of saliva: current state and future applications. Clin. Chem. 57, 675–687 (2011).
Javaid, M. A., Ahmed, A. S., Durand, R. & Tran, S. D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 6, 66–75 (2016).
Goswami, Y., Mishra, R., Agrawal, A. P. & Agrawal, L. A. Salivary biomarkers: a review of powerful diagnostic tool. IOSR J. Dent. Med. Sci. 14, 80–87 (2015).
Campuzano, S., Yanez-Sedeno, P. & Pingarron, J. M. Electrochemical bioaffinity sensors for salivary biomarkers detection. Trends Analyt. Chem. 86, 14–24 (2017).
Soni, A. & Jha, S. K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 67, 763–768 (2015).
Stevens, R. C., Soelberg, S. D., Near, S. & Furlong C.E. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem. 80, 6747–6751 (2008).
Yamaguchi, M. et al. Hand-held monitor of sympathetic nervous system using salivary amylase activity and its validation by driver fatigue assessment. Biosens. Bioelectron. 21, 1007–1014 (2006).
Ballesta Claver, J., Valencia Mirón, M. C. & Capitán-Vallvey, L. F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst 134, 1423–1432 (2009).
Zangheri, M. et al. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 64, 63–68 (2015).
Mahosenaho, M. et al. A disposable biosensor for the determination of alpha-amylase in human saliva. Mikrochim. Acta 170, 243–249 (2010).
Soni, A. & Jha, S. K. Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta 996, 54–63 (2017).
Zhang, L., Yang, W., Yang, Y., Liu, H. & Gu, Z. Smartphone-based point-of-care testing of salivary α-amylase for personal psychological measurement. Analyst 140, 7399–7406 (2015).
Roda, A. et al. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 139, 6494–6501 (2014).
Aguirre, A. et al. Sialochemistry: a diagnostic tool? Crit. Rev. Oral Biol. Med. 4, 343–350 (1993).
Kaufman, E. & Lamster, I. B. The diagnostic applications of saliva—a review. Crit. Rev. Oral Biol. Med. 13, 197–212 (2002).
Chiappin, S., Antonelli, G., Gatti, R. & De Palo, E. F. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 383, 30–40 (2007).
Streckfus, C. F. & Bigler, L. R. Saliva as a diagnostic fluid. Oral Dis. 8, 69–76 (2002).
Mandel, I. D. The diagnostic uses of saliva. J. Oral Pathol. Med. 19, 119–125 (1990).
Malon, R. S. P., Sadir, S., Balakrishnan, M. & Córcoles, E. P. Saliva-based biosensors: noninvasive monitoring tool for clinical diagnostics. Biomed Res. Int. 2014, 962903 (2014).
Chicharro, J. L., Lucía, A., Pérez, M., Vaquero, A. F. & Ureña, R. Saliva composition and exercise. Sports Med. 26, 17–27 (1998).
Singh, M. Non invasive diagnostic tool for pathological conditions salivary biomarkers. Int. J. Pharm. Biol. Arch. 5, 1–12 (2014).
Gatti, R. & De Palo, E. F. An update: salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 21, 157–169 (2011).
Viswanath, B., Choi, C. S., Lee, K. & Kim, S. Recent trends in the development of diagnostic tools for diabetes mellitus using patient saliva. Trends Analyt. Chem. 89, 60–67 (2017).
Kim, J. et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139, 1632–1636 (2014).
Segura, R. et al. A new approach to the assessment of anaerobic metabolism: measurement of lactate in saliva. Br. J. Sports Med. 30, 305–309 (1996).
Santos, R. V. T., Almeida, A. L. R., Caperuto, E. C., Martins, E. Jr. & Costa Rosa, L. F. B. P. Effects of a 30-km race upon salivary lactate correlation with blood lactate. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 145, 114–117 (2006).
Arakawa, T. et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: a novel cavitas sensor. Biosens. Bioelectron. 84, 106–111 (2016).
Yamaguchi, M., Mitsumori, M. & Kano, Y. Noninvasively measuring blood glucose using saliva. IEEE Eng. Med. Biol. Mag. 17, 59–63 (1998).
Agrawal, R. et al. Noninvasive method for glucose level estimation by saliva. J. Diabetes Metab. 4, 2–5 (2013).
Zhang, W., Du, Y. & Wang, M. L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Biosensing Res. 4, 23–29 (2015).
Jurysta, C. et al. Salivary glucose concentration and excretion in normal and diabetic subjects. J. Biomed. Biotechnol. 2009, 430426 (2009).
Campbell, M. J. A. Glucose in the saliva of the non-diabetic and the diabetic patient. Arch. Oral Biol. 10, 197–205 (1965).
Abikshyeet, P., Ramesh, V. & Oza, N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab. Syndr. Obes. 5, 149–154 (2012).
Tseng, P., Napier, B., Garbarini, L., Kaplan, D. L. & Omenetto, F. G. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater. 30, e1703257 (2018).
Lee, Y. et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl Acad. Sci. USA 115, 5377–5382 (2018).
Sarpeshkar, R. Universal principles for ultra low power and energy efficient design. IEEE Trans. Circuits Syst. II 59, 193–198 (2012).
De Marcellis, A., Depari, A., Ferri, G., Flammini, A. & Sisinni, E. A CMOS integrated low-voltage low-power time-controlled interface for chemical resistive sensors. Sens. Actuators B Chem. 179, 313–318 (2013).
Zhang, Y. et al. Realizing both high energy and high power densities by twisting three carbon-nanotube-based hybrid fibers. Angew. Chem. Int. Ed. Engl. 54, 11177–11182 (2015).
Huang, Y. et al. Weavable, conductive yarn-based NiCo//Zn textile battery with high energy density and rate capability. ACS Nano 11, 8953–8961 (2017).
Mesin, L. A neural algorithm for the non-uniform and adaptive sampling of biomedical data. Comput. Biol. Med. 71, 223–230 (2016).
Zi, Y. et al. Triboelectric-pyroelectric-piezoelectric hybrid cell for high-efficiency energy-harvesting and self-powered sensing. Adv. Mater. 27, 2340–2347 (2015).
Zhang, K. & Yang, Y. Thermo-phototronic effect enhanced InP/ZnO nanorod heterojunction solar cells for self-powered wearable electronics. Adv. Funct. Mater. 27, 1703331 (2017).
Wen, Z. et al. Self-powered textile for wearable electronics by hybridizing fiber-shaped nanogenerators, solar cells, and supercapacitors. Sci. Adv. 2, e1600097 (2016).
Bandodkar, A. J. et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 10, 1581–1589 (2017).
Ha, M., Park, J., Lee, Y. & Ko, H. Triboelectric generators and sensors for self-powered wearable electronics. ACS Nano 9, 3421–3427 (2015).
Kim, C.S. et al. Self-powered wearable electrocardiography using a wearable thermoelectric power generator. ACS Energy Lett. 3, 501–507 (2018).
Imani, S., Mercier, P.P., Bandodkar, A.J., Kim, J. & Wang, J. in 2016 IEEE International Symposium on Circuits and Systems (ISCAS) 1122–1125 (IEEE, 2016).
Khan, S.U., Zomaya, A.Y. & Abbas, A. Handbook of Large-Scale Distributed Computing in Smart Healthcare (Springer, 2017).
Li, M., Lou, W. & Ren, K. Data security and privacy in wireless body area networks. IEEE Wirel. Commun. 17, 51–58 (2010).
Kotz, D., Gunter, C. A., Kumar, S. & Weiner, J. P. Privacy and security in mobile health: a research agenda. Computer 49, 22–30 (2016).
Dimitriou, T. & Ioannis, K. in First International Symposium on Applied Sciences on Biomedical and Communication Technologies, 2008 ISABEL’08 1–5 (IEEE, 2008).
Daniels, J. S. & Pourmand, N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis 19, 1239–1257 (2007).
Xiao, Y., Lubin, A. A., Heeger, A. J. & Plaxco, K. W. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. Int. Ed. Engl. 44, 5456–5459 (2005).
Hammami, A., Raymond, N. & Armand, M. Lithium-ion batteries: runaway risk of forming toxic compounds. Nature 424, 635–636 (2003).
Lisbona, D. & Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 89, 434–442 (2011).
Xu, S. et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 4, 1543 (2013).
Berchmans, S. et al. An epidermal alkaline rechargeable Ag-Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A Mater. 2, 15788–15795 (2014).
Kumar, R. et al. All-printed, stretchable Zn-Ag2O rechargeable battery via hyperelastic binder for self-powering wearable electronics. Adv. Energy Mater. 7, 1–8 (2017).
Qiu, Y. C. et al. Vertically aligned carbon nanotubes on carbon nanofibers: a hierarchical three-dimensional carbon nanostructure for high-energy flexible supercapacitors. Chem. Mater. 27, 1194–1200 (2015).
Li, Q., Lu, X. F., Xu, H., Tong, Y. X. & Li, G. R. Carbon/MnO2 double-walled nanotube arrays with fast ion and electron transmission for high-performance supercapacitors. ACS Appl. Mater. Interfaces 6, 2726–2733 (2014).
Wang, Q., Yan, J. & Fan, Z. J. Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities. Energy Environ. Sci. 9, 729–762 (2016).
O’Connor, T. F. et al. Wearable organic solar cells with high cyclic bending stability: materials selection criteria. Sol. Energy Mater. Sol. Cells 144, 438–444 (2016).
Kim, B. J. et al. Highly efficient and bending durable perovskite solar cells: toward a wearable power source. Energy Environ. Sci. 8, 916–921 (2015).
Yang, J.H. et al. Effect of garment design on piezoelectricity harvesting from joint movement. Smart Mater. Struct. 25, 1–15 (2016).
Pu, X. et al. Wearable self-charging power textile based on flexible yarn supercapacitors and fabric nanogenerators. Adv. Mater. 28, 98–105 (2016).
Oh, J. Y. et al. Chemically exfoliated transition metal dichalcogenide nanosheet-based wearable thermoelectric generators. Energy Environ. Sci. 9, 1696–1705 (2016).
Lu, Z. S., Zhang, H. H., Mao, C. P. & Li, C. M. Silk fabric-based wearable thermoelectric generator for energy harvesting from the human body. Appl. Energy 164, 57–63 (2016).
Jia, W., Valdés-Ramírez, G., Bandodkar, A. J., Windmiller, J. R. & Wang, J. Epidermal biofuel cells: energy harvesting from human perspiration. Angew. Chem. Int. Ed. Engl. 52, 7233–7236 (2013).
Jia, W. Z. et al. Wearable textile biofuel cells for powering electronics. J. Mater. Chem. A Mater. 2, 18184–18189 (2014).
Bandodkar, A. J. & Wang, J. Wearable biofuel cells: a review. Electroanalysis 28, 1188–1200 (2016).
Ogawa, Y. et al. Stretchable biofuel cell with enzyme-modified conductive textiles. Biosens. Bioelectron. 74, 947–952 (2015).
Falk, M., Andoralov, V., Silow, M., Toscano, M. D. & Shleev, S. Miniature biofuel cell as a potential power source for glucose-sensing contact lenses. Anal. Chem. 85, 6342–6348 (2013).
Grattieri, M. & Minteer, S. D. Self-powered biosensors. ACS Sens. 3, 44–53 (2018).
Yetisen, A. K., Martinez-Hurtado, J. L., Garcia-Melendrez, A., Vasconcellos, F. D. & Lowe, C. R. A smartphone algorithm with inter-phone repeatability for the analysis of colorimetric tests. Sens. Actuators B Chem. 196, 156–160 (2014).
Acknowledgements
This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (HDTRA 1-16-1-0013). A.S.C. acknowledges funding through NIH NIAAA T32 Training Grant AA013525.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J., Campbell, A.S., de Ávila, B.EF. et al. Wearable biosensors for healthcare monitoring. Nat Biotechnol 37, 389–406 (2019). https://doi.org/10.1038/s41587-019-0045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0045-y
This article is cited by
-
Recent advances in two-dimensional nanomaterials for sustainable wearable electronic devices
Journal of Nanobiotechnology (2024)
-
Using body sensors for evaluating the impact of smart cycling technologies on cycling experiences: a systematic literature review and conceptual framework
European Transport Research Review (2024)
-
Towards on-skin analysis of sweat for managing disorders of substance abuse
Nature Biomedical Engineering (2024)
-
Ultrasensitive detection of vital biomolecules based on a multi-purpose BioMEMS for Point of care testing: digoxin measurement as a case study
Scientific Reports (2024)
-
High-order sensory processing nanocircuit based on coupled VO2 oscillators
Nature Communications (2024)