Abstract
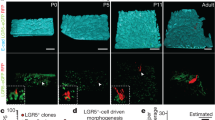
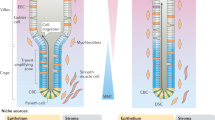
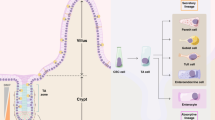
The morphology and functionality of the epithelial lining differ along the intestinal tract, but tissue renewal at all sites is driven by stem cells at the base of crypts1,2,3. Whether stem cell numbers and behaviour vary at different sites is unknown. Here we show using intravital microscopy that, despite similarities in the number and distribution of proliferative cells with an Lgr5 signature in mice, small intestinal crypts contain twice as many effective stem cells as large intestinal crypts. We find that, although passively displaced by a conveyor-belt-like upward movement, small intestinal cells positioned away from the crypt base can function as long-term effective stem cells owing to Wnt-dependent retrograde cellular movement. By contrast, the near absence of retrograde movement in the large intestine restricts cell repositioning, leading to a reduction in effective stem cell number. Moreover, after suppression of the retrograde movement in the small intestine, the number of effective stem cells is reduced, and the rate of monoclonal conversion of crypts is accelerated. Together, these results show that the number of effective stem cells is determined by active retrograde movement, revealing a new channel of stem cell regulation that can be experimentally and pharmacologically manipulated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq data are available at the Gene Expression Omnibus (GEO) under accession code GSE194250. All other data are included within the Article and its Extended Data and Supplementary Information and are available at Figshare (https://figshare.com/projects/Azkanaz_et_al_2022_Retrograde_movements_determine_effective_stem_cell_numbers_in_the_intestine/139210). Source data are provided with this paper.
Code availability
The codes and data used for fits, simulations, RNA-seq, in vitro cell migration and single LGR5+ cell migration on dECM are available at GitHub (https://github.com/JaccovanRheenenLab/Retrograde_movement_Azkanaz_Nature_2022).
References
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Breault, D. T. et al. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc. Natl Acad. Sci. USA 105, 10420–10425 (2008).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 (2008).
Takeda, N. et al. Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 (2011).
Barriga, F. M. et al. Mex3a marks a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell 20, 801–816 (2017).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Kozar, S. et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13, 626–633 (2013).
Corominas-Murtra, B. et al. Stem cell lineage survival as a noisy competition for niche access. Proc. Natl Acad. Sci. USA 117, 16969–16975 (2020).
Hu, D. J., Yun, J., Elstrott, J. & Jasper, H. Non-canonical Wnt signaling promotes directed migration of intestinal stem cells to sites of injury. Nat. Commun. 12, 7150 (2021).
Gregorieff, A. et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638 (2005).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Iqbal, S. et al. Fetal-like reversion in the regenerating intestine is regulated by mesenchymal Asporin. Preprint at bioRxiv https://doi.org/10.1101/2021.06.24.449590 (2021).
Huels, D. J. et al. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat. Commun. 9, 1132 (2018).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Durand, A. et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc. Natl Acad. Sci. USA 109, 8965–8970 (2012).
Kim, T. H., Escudero, S. & Shivdasani, R. A. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc. Natl Acad. Sci. USA 109, 3932–3937 (2012).
Pentinmikko, N. et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402 (2019).
Flanagan, D. J. et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature 594, 430–435 (2021).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Ritsma, L. et al. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 8, 583–594 (2013).
McLean, I. W. & Nakane, P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22, 1077–1083 (1974).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Jiang, H., Lei, R., Ding, S. W. & Zhu, S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 15, 182 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Tinevez, J. Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Serra, D. et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72 (2019).
Miao, Y. et al. Next-generation surrogate Wnts support organoid growth and deconvolute frizzled pleiotropy in vivo. Cell Stem Cell 27, 840–851 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Wortel, I. M. N., Dannenberg, K., Berry, J. C., Miller, M. J. & Textor, J. CelltrackR: an R package for fast and flexible analysis of immune cell migration data. Preprint at bioRxiv https://doi.org/10.1101/670505 (2019).
Gorelik, R. & Gautreau, A. Quantitative and unbiased analysis of directional persistence in cell migration. Nat. Protoc. 9, 1931–1943 (2014).
Acknowledgements
We thank the members of the van Rheenen laboratory for reading the manuscript, and the members of the bioimaging, FACS and animal facility of the NKI for experimental support. We acknowledge the staff at the MedH Flow Cytometry core facility, Karolinska Institutet, and LCI facility/Nikon Center of Excellence, Karolinska Institutet. This work was financially supported by the Netherlands Organization of Scientific Research NWO (Veni grant 863.15.011 to S.I.J.E. and Vici grant 09150182110004 to J.v.R.) and the CancerGenomics.nl (Netherlands Organisation for Scientific Research) program (to J.v.R.) the Doctor Josef Steiner Foundation (to J.v.R). B.D.S. acknowledges funding from the Royal Society E.P. Abraham Research Professorship (RP\R1\180165) and the Wellcome Trust (098357/Z/12/Z and 219478/Z/19/Z). B.C.-M. acknowledges the support of the field of excellence ‘Complexity of life in basic research and innovation’ of the University of Graz. O.J.S. and their laboratory acknowledge CRUK core funding to the CRUK Beatson Institute (A17196 and A31287) and CRUK core funding to the Sansom laboratory (A21139). P.K. and their laboratory are supported by grants from the Swedish Research Council (2018-03078), Cancerfonden (190634), Academy of Finland Centre of Excellence (266869, 304591 and 320185) and the Jane and Aatos Erkko Foundation. P.L. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 758617). E.H. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 851288).
Author information
Authors and Affiliations
Contributions
S.I.J.E., L.B., E.H., B.D.S., A.T.W., P.K. and J.v.R. conceived parts of the study. M.A., S.I.J.E., L.B. and S.J.A.L. performed the intravital microscopy experiments with the supervision of H.J.S. and J.v.R. B.C.-M. and E.H. performed the mathematical modelling with input from B.D.S. D.J.H., D.J.F. and O.J.S. provided in situ hybridization, immunohistochemistry and LGK974-treatment data. A.T.W., S.I., K.A., M.K. and P.K. developed and performed the decellularization experiments and the single-cell motility analysis. K.C.O. and P.L. performed the in vitro cell migration assays. D.L. performed analysis of the in vitro cell migration and sequencing data. F.R.-R. helped with analysing and interpreting sequencing data. M.V. and H.A.M. validated data by qPCR and imaging. All of the authors contributed to writing and have approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Elaine Fuchs, Christina Lo Celso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Crypt characteristics in small and large intestine.
a, Quantification of the number of LGR5+ cells per position in crypt of SI (n = 12 crypts) and LI (n = 12 crypts) in Lgr5eGFP-Ires-CreERT2 mice. Mean +/− SD are plotted b, Schematic representation of experimental setup for RNA-seq and organoid forming assay. c,d, Volcano plots showing log2 fold-change (x-axis) and -Log10 p-value (y-axis) of genes differentially expressed between LGR5+ cells with medium intensity (border) and LGR5+ cells with high intensity (centre). Genes that were significantly altered in border compared with centre LGR5+ cells are highlighted in red (Log2 fold change >2, -Log10 p-value <0.001) in SI (c) and LI (d), n = 4 mice for each condition. e, Stem cell markers (Lgr5, Ascl2 and Smoc2) in situ hybridization (ISH) in C57/B6 mouse SI (top) and LI (bottom) crypts, n = 4 mice. Scale bar, 100 μm. f, Wnt targets (AXIN2, CD44, CYCD1) ISH and immunohistochemistry (IHC) in C57/B6 mouse SI (top) and LI (bottom) crypts, n = 4 mice. Scale bar, 100 μm. g, Immunofluorescence (IF) staining of Ephrin B2 and Ephrin B3 in C57/B6 mouse SI (top) and LI (bottom) crypts, n = 3 experiments. Scale bar, 20 μm. h, Confocal images of isolated crypts (dotted outline) of SI (left), and LI (right), proliferating cells were identified by BrdU incorporation upon 2-hour pulse (red). Nuclei were labelled using DAPI (blue), n = 10 experiments. Scale bar, 50 μm.
Extended Data Fig. 2 Visualizing effective stem cells by intravital imaging in small and large intestine.
a,b, Representative overview images 48 h (left) and 8 weeks (right) after tracing in Lgr5eGFP-Ires-CreERT2 mice from 6 independent experiments. Dotted lines represent same areas. Grey lines indicate SI LI boundary. Lower pictures represent intravital images showing crypt patterns (LGR5-eGFP in green) at 48h and 8w after tracing in SI (a) and LI (b). Dotted lines are examples of retraced patchy LGR5+ areas. Scale bar, 5 mm (top), 100 μm (bottom) from 5 independent experiments. c,d, Quantification of retention within the LGR5+ zone of clones starting from different positions in the niche (shades of green) in SI (c) and LI (d) as followed by IVM. SI: n = 305 clones in 9 mice; LI: n = 311 clones in 5 mice (see Ext. Data Fig. 3–6).
Extended Data Fig. 3 Short-term evolution of clones in SI (1).
Presence in centre (light green) and border (dark green) of individual clones in Lgr5eGFP-Ires-CreERT2; R26-Confetti mice followed by short-term IVM in SI is plotted over time (squares represent individual clones, with a bar per day). Plotted are clones starting and remaining in the centre (top panel), starting and remaining in centre while spreading to border (middle panel) and starting in the centre and transferring to border (bottom panel).
Extended Data Fig. 4 Short-term evolution of clones in SI (2).
Presence in centre (light green) and border (dark green) of individual clones in Lgr5eGFP-Ires-CreERT2; R26-Confetti mice followed by short-term IVM in SI are plotted over time (represent individual clones with a bar per day). Plotted are clones starting in the centre and getting lost (top panel), starting in border and transferring to centre (second panel), starting in border and remaining in border, and starting in border before getting lost.
Extended Data Fig. 5 Short-term evolution of clones in LI (1).
Presence in centre (light green) and border (dark green) of individual clones in Lgr5eGFP-Ires-CreERT2; R26-Confetti mice followed by short-term IVM in LI are plotted over time (squares represent individual clones with a bar per day). Plotted are clones starting and remaining in the centre while spreading to border (top panel), starting in the centre and transferring to border (second panel), starting and remaining in centre without spreading to border (third panel) and starting in the centre before getting lost from the niche (bottom panel).
Extended Data Fig. 6 Short-term evolution of clones in LI (2).
Presence in centre (light green) and border (dark green) of individual clones in Lgr5eGFP-Ires-CreERT2; R26-Confetti mice followed by short-term IVM in LI are plotted over time (squares represent individual clones with a bar per day). Clones are Plotted are clones starting in border and transferring to centre (top panel), starting in border and remaining there (second panel), starting in border before getting lost (bottom panel).
Extended Data Fig. 7 Wnt enhances motility in vitro.
a, Percentage of fast-moving (>2 µm/min), slow-moving (0.3-2 µm/min) and non-moving (<0.3 µm/min) LGR5+ cells. The imaged LGR5+ cells were isolated from Lgr5-EGFP-ires-creERT2;R26R-confetti organoids and exposed to (I) control medium (n = 408 cells), (II) medium supplemented with Wnt3a (n = 582 cells), (III) medium supplemented with Paneth cells (PC) (n = 418 cells), or (IV) medium supplemented with PC and Wnt inhibitor (IWP2) (n = 431 cells) in Matrigel from 3 independent biological replicates. b,c Speed (b) and directionality ratio (persistence) over time calculated as mean displacement/length of the trajectory. Significance was determined by a two-sided Mann-Whitney test.(c) of single LGR5+ cells in control medium, medium supplemented with Wnt3a, co-culture with PC and co-culture PC with IWP2. Shown are n = 150 random cell tracks of LGR5+ cells from 2 independent organoid lines, 50 from each of 3 independent biological replicates. Each point represents the mean value of each track. Shown are mean ± SEM.
Extended Data Fig. 8 The effect of LGK974 on stem cell dynamics in small intestinal crypts.
a, Representative image of 2h BrdU pulse in SI crypts of control and LGK974-treated mice. Scale bar, 50 μm. b, Quantification of cells positive for BrdU per position in SI crypts of control and LGK974-treated mice. Of note, position is based on nuclei count which does not discriminate between stem cells and PCs, and the LGR5+ zone ends around nuclear position 6-8. Mean +/− SEM are plotted. (n = 120 crypts examined over 4 independent experiments from 4 mice, 30 crypts per mouse).
Extended Data Fig. 9 Biophysical modelling of stochastic conveyor belt dynamics in small versus large intestine.
a, An intestinal crypt is abstracted as a hemispherical surface. A cell experiences net upwards force due to the divisions taking place at lower positions, together with stochastic repositioning events. b, This hemispheric region can be segmented by cuts at different heights, ℓ0, ℓ1, ℓ2, ℓ3. c, If the sections defined by these cuts are of the same width, 𝛥ℓ, then the area of each is the same, which provides an explanation for the near-constant number of LGR5+ cell at each position. d, This allows us to approximate the system as consecutive layers of cells on a cylinder. e, Analytical solutions for the stochastic conveyor belt model (probability of clone retention per time). Left (resp. right) plot shows the retention probability as a function of the starting position of the mother cell of the lineage for the SI (resp. LI). Points show the experimental data for wild-type (same as Fig. 3), lines are the prediction of the stochastic conveyer belt dynamics given by equation (1.3) of the SI Theory Note. In both panels, the color scheme is: Green, 2 days, Blue, 3 days, orange, 4 days, and red 56 days post-labelling. f, Average monoclonal conversion in crypts for different values of 𝑘𝑟 𝑘𝑑 ; and rescaled time it takes to convert. g, Corresponding time of conversion as a function of 𝑘𝑟 𝑘𝑑 (points) which are very well fitted by a square root (lines), showing that the time increases close to linearly with \(\surd \)𝑘𝑟/𝑘𝑑. h, Sensitivity analysis of the 2D numerical simulations. Top, effect of increasing values of the division rate kd on the resulting short-term clonal retention dynamics as a function of initial cell positions at days 2, 3 and 4 (left, middle and right panel respectively), for constant kr = 0.25 (LI best-fit value). Increasing thickness of the lines indicate increasing division rate (or alternatively decreasing division time: 2.3, 1.7, 1.4, 1.2, 0.9 divisions per day respectively – note that the middle curve thus corresponds to the value of 1.4 divisions per day used in the main text). Bottom, Effect of increasing values of the division rate kr on the resulting short-term clonal retention dynamics as a function of initial cell positions at days 2, 3 and 4 (left, middle and right panel respectively), for constant kd = 0.5 (LI best-fit value). Increasing thickness of the lines indicate increasing kr = 0.25,1, 2, 3, 4 (note that the first curve thus corresponds to the best fit value used in the main text). i, Comparison between 1D analytical theory (solid lines) and 2D simulations (circles) for the clonal retention probability (y-axis, parameters chosen as kr = 2, 1/kd = 1.2 divisions per day) as a function of initial starting position for the clone (x-axis) and time (colors red, green, blue, yellow and red indicating resp. day 1, day 2, day 3, day 4 and day 56). Dashed region indicates the standard deviation observed in the simulations for the respective simulation time. j,k, Normalized probability of retention in LGR5+ zone for different starting positions over time in SI (f; n = 305 clones in 9 mice) and LI (g; n = 334 clones in 5 mice) predicted by model (solid lines and shaded intervals, mean with 95% confidence interval) and experimental data (dots). l,m, Probability of presence in centre, border or loss of centre-starting (left) and border-starting clones (right) over time in SI (h, n = 305 clones in 9 mice) and LI (I, n = 311 clones in 5 mice), comparing data (left bar) and theory (right bar).
Extended Data Fig. 10 Clonal dispersion in small and large intestine.
a, Typical outputs of 2D numerical simulations of a single clonal labelling event (labelled cells indicated in red) for the parameter set extracted from SI (left) and LI (right) data. As expected, larger values of kr result in a higher probability of clonal fragmentation (defined as the probability of a given clone displaying two fragments separated by a row of clonally non-labelled cells, see SI Note for details on the simulations). b, SI (top) and LI (bottom) crypts with sparse lineage-tracing experiment, where a single lineage (red here, induced and imaged 7 days post induction) can be observed. Clonal dispersion due to cell rearrangements is either observed (right) or not (left). Scale bar, 20 μm. c, Probability of clonal fragmentation in SI and LI (data shown in grey (SI) and black (LI), theory in dotted bars extracted from the parameters in panel a), showing good agreement. Data is based on n = 3 mice (20 crypts for SI and 55 crypts for LI). Each data point represents percentage of clonal dispersed crypts in one mouse, and bars show mean +/− SD.
Supplementary information
Supplementary Information
Supplementary Note 1: basics of stochastic conveyor belt (SCB) dynamics; Supplementary Note 2: numerical simulations of the 2D SCB dynamics and statistics; Supplementary Note 3: comparison with previous models; and Supplementary References.
Supplementary Figure 1
FACS gating strategy. Gating strategy to isolate single LGR5+ cells with high, medium and low intensity corresponding to centre, border and >3 row cells from dissociated intestinal crypts (corresponding to Fig 1h–j and Extended Data Fig. 1b–d). The percentage of parent population is shown.
Supplementary Video 1
Wnt induces migration of LGR5+ cells. Migration of LGR5+confetti–RFP+ (red) in various indicated conditions. The tracks of migration are indicated by coloured lines. PCs are are shown in yellow. Time is depicted in hours. Scale bar, 20 μm.
Supplementary Video 2
Active retrograde movement of LGR5+ cells on a decellularized intestinal scaffold. The video shows the movement of LGR5+ cell (red arrows and square) towards the base of the crypt. Left, cartoon and still images of LGR5+ cell (in green), ColF (in grey), tracking the cell and the respective localization in Z and in respect to the villi and crypts. Right, a video showing different colours for different Z-levels of the LGR5+ cell as indicated by the lookup table (LUT) in the left cartoon. The given cell traverses 48 µm distance in to the lower part of the crypt.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azkanaz, M., Corominas-Murtra, B., Ellenbroek, S.I.J. et al. Retrograde movements determine effective stem cell numbers in the intestine. Nature 607, 548–554 (2022). https://doi.org/10.1038/s41586-022-04962-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04962-0
This article is cited by
-
Effects of hormones on intestinal stem cells
Stem Cell Research & Therapy (2023)
-
Yo-yoing stem cells defy dogma to maintain hair colour
Nature (2023)
-
Intracellular pH dynamics regulates intestinal stem cell lineage specification
Nature Communications (2023)
-
Advanced Progression for the Heterogeneity and Homeostasis of Intestinal Stem Cells
Stem Cell Reviews and Reports (2023)
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.