Abstract
Ageing is intimately connected to the induction of cell senescence1,2, but why this is so remains poorly understood. A key challenge is the identification of pathways that normally suppress senescence, are lost during ageing and are functionally relevant to oppose ageing3. Here we connected the structural and functional decline of ageing tissues to attenuated function of the master effectors of cellular mechanosignalling YAP and TAZ. YAP/TAZ activity declines during physiological ageing in stromal cells, and mimicking such decline through genetic inactivation of YAP/TAZ in these cells leads to accelerated ageing. Conversely, sustaining YAP function rejuvenates old cells and opposes the emergence of ageing-related traits associated with either physiological ageing or accelerated ageing triggered by a mechano-defective extracellular matrix. Ageing traits induced by inactivation of YAP/TAZ are preceded by induction of tissue senescence. This occurs because YAP/TAZ mechanotransduction suppresses cGAS–STING signalling, to the extent that inhibition of STING prevents tissue senescence and premature ageing-related tissue degeneration after YAP/TAZ inactivation. Mechanistically, YAP/TAZ-mediated control of cGAS–STING signalling relies on the unexpected role of YAP/TAZ in preserving nuclear envelope integrity, at least in part through direct transcriptional regulation of lamin B1 and ACTR2, the latter of which is involved in building the peri-nuclear actin cap. The findings demonstrate that declining YAP/TAZ mechanotransduction drives ageing by unleashing cGAS–STING signalling, a pillar of innate immunity. Thus, sustaining YAP/TAZ mechanosignalling or inhibiting STING may represent promising approaches for limiting senescence-associated inflammation and improving healthy ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All RNA-seq and scRNA-seq data generated for the present study are available as GSE165668 at the Gene-Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). scRNA-seq data from the Tabula MurisSenis atlas were obtained from the Gene-Expression Omnibus (GSE149590). Raw scRNA-seq data of mouse SSCs were downloaded from the Gene-Expression Omnibus (GSE161946). Source data are provided with this paper.
Code availability
All code was performed using R v.3.5.0 and publicly available packages explicitly cited in the manuscript. No custom functions were written for the analysis. STAR is available from GitHub (https://github.com/alexdobin/STAR).
References
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Ewald, C. Y. The matrisome during aging and longevity: a systems-level approach toward defining matreotypes promoting healthy aging. Gerontology 66, 266–274 (2020).
Ge, Y. et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl Acad. Sci. USA 117, 5339–5350 (2020).
Panciera, T., Azzolin, L., Cordenonsi, M. & Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 18, 758–770 (2017).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Bio. 20, 457–473 (2019).
Meng, Z. et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature 560, 655–660 (2018).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Tabula Muris, C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Tigges, J. et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 138, 26–44 (2014).
Humphrey, J. D., Milewicz, D. M., Tellides, G. & Schwartz, M. A. Cell biology. Dysfunctional mechanosensing in aneurysms. Science 344, 477–479 (2014).
Zheng, B., Zhang, Z., Black, C. M., de Crombrugghe, B. & Denton, C. P. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am. J. Pathol. 160, 1609–1617 (2002).
Fisher, G. J., Varani, J. & Voorhees, J. J. Looking older: fibroblast collapse and therapeutic implications. Arch. Dermatol. 144, 666–672 (2008).
Tchkonia, T. et al. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 (2010).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007).
Wirth, A. et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 (2008).
Sengle, G. & Sakai, L. Y. The fibrillin microfibril scaffold: a niche for growth factors and mechanosensation? Matrix Biol. 47, 3–12 (2015).
Salvi, P. et al. Aortic dilatation in Marfan syndrome: role of arterial stiffness and fibrillin-1 variants. J. Hypertens. 36, 77–84 (2018).
Bunton, T. E. et al. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ. Res. 88, 37–43 (2001).
Santinon, G. et al. dNTP metabolism links mechanical cues and YAP/TAZ to cell growth and oncogene-induced senescence. EMBO J. 37, e97780 (2018).
Xu, Y. et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl Acad. Sci. USA 107, 8129–8134 (2010).
Dou, Z. et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Ablasser, A. & Chen, Z. J. cGAS in action: expanding roles in immunity and inflammation. Science https://doi.org/10.1126/science.aat8657 (2019).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Sauer, J. D. et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79, 688–694 (2011).
Haag, S. M. et al. Targeting STING with covalent small-molecule inhibitors. Nature 559, 269–273 (2018).
Larrieu, D., Britton, S., Demir, M., Rodriguez, R. & Jackson, S. P. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344, 527–532 (2014).
Khatau, S. B. et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl Acad. Sci. USA 106, 19017–19022 (2009).
Kim, J. K. et al. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 8, 2123 (2017).
Maurer, M. & Lammerding, J. The driving force: nuclear mechanotransduction in cellular function, fate, and disease. Annu. Rev. Biomed. Eng. 21, 443–468 (2019).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410 e1314 (2017).
Vergnes, L., Peterfy, M., Bergo, M. O., Young, S. G. & Reue, K. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl Acad. Sci. USA 101, 10428–10433 (2004).
Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25, 2579–2593 (2011).
Bedrosian, T. A. et al. Lamin B1 decline underlies age-related loss of adult hippocampal neurogenesis. EMBO J. 40, e105819 (2021).
Dreesen, O. et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 200, 605–617 (2013).
Shah, P. P. et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013).
Goley, E. D. & Welch, M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726 (2006).
Dang, I. et al. Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature 503, 281–284 (2013).
Totaro, A. et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat. Commun. 8, 15206 (2017).
Nader, G. P. F. et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell https://doi.org/10.1016/j.cell.2021.08.035 (2021).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Kidiyoor, G. R. et al. ATR is essential for preservation of cell mechanics and nuclear integrity during interstitial migration. Nat. Commun. 11, 4828 (2020).
He, C. et al. YAP1-LATS2 feedback loop dictates senescent or malignant cell fate to maintain tissue homeostasis. EMBO Rep. https://doi.org/10.15252/embr.201744948 (2019).
Fausti, F. et al. ATM kinase enables the functional axis of YAP, PML and p53 to ameliorate loss of Werner protein-mediated oncogenic senescence. Cell Death Differ. 20, 1498–1509 (2013).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016).
Gurkar, A. U. & Niedernhofer, L. J. Comparison of mice with accelerated aging caused by distinct mechanisms. Exp. Gerontol. 68, 43–50 (2015).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Judge, D. P. et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 114, 172–181 (2004).
Azzolin, L. et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014).
Totaro, A. et al. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc. Natl Acad. Sci. USA 116, 17848–17857 (2019).
Morsut, L. et al. Negative control of Smad activity by ectodermin/Tif1gamma patterns the mammalian embryo. Development 137, 2571–2578 (2010).
Panciera, T. et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat. Mater. 19, 797–806 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e1821 (2019).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291 e289 (2019).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Morikawa, Y. et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci. Signal 8, ra41 (2015).
Hetrick, B., Han, M. S., Helgeson, L. A. & Nolen, B. J. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol. 20, 701–712 (2013).
Panciera, T. et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA 114, E4612–E4620 (2017).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Haydont, V., Neiveyans, V., Zucchi, H., Fortunel, N. O. & Asselineau, D. Genome-wide profiling of adult human papillary and reticular fibroblasts identifies ACAN, Col XI alpha1, and PSG1 as general biomarkers of dermis ageing, and KANK4 as an exemplary effector of papillary fibroblast ageing, related to contractility. Mech. Ageing Dev. 177, 157–181 (2019).
Haydont, V., Neiveyans, V., Fortunel, N. O. & Asselineau, D. Transcriptome profiling of human papillary and reticular fibroblasts from adult interfollicular dermis pinpoints the 'tissue skeleton’ gene network as a component of skin chrono-ageing. Mech. Ageing Dev. 179, 60–77 (2019).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019).
Mahmoudi, S. et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574, 553–558 (2019).
Salzer, M. C. et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175, 1575–1590 e1522 (2018).
Waldera Lupa, D. M. et al. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J. Invest. Dermatol. 135, 1954–1968 (2015).
Zanconato, F. et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat. Med. 24, 1599–1610 (2018).
Stein, C. et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 11, e1005465 (2015).
Acknowledgements
We thank colleagues sharing their plasmids on Addgene, the IFOM imaging facility team (D. Parazzoli, M. Garre, A. Oldani, G. Beznusenko and A. Mironov) for advice on image processing; S. Soligo, I. D’Arsiè and B. Marchesan for managing the mouse colony, and all members of our group for reading the manuscript. We are grateful to V. Cancila for support on PLA and M. Sandri and L. Vitiello for providing C57BL/6N WT mice older than 18 months. H.L.S.-M. was first supported by grant no. EMBO ALTF 987-2016 and then by Marie Skłodowska-Curie Individual Fellowship grant no. 798570-YAP MECHANO; G. Brumana was supported by a Fondazione Cariparo PhD fellowship. The research leading to these results has received funding from the following agencies/charities: Fondazione AIRC under 5 per mille 2019 - ID. 22759 programme, P.I. S.P.; Fondazione AIRC, IG 2019 ID. 23307 project, P.I. S.P.; the European Research Council under the European Union’s Horizon 2020 research and innovation programme DENOVOSTEM grant agreement no. 670126 to S.P.; PRIN-MIUR to S.P., T.P. and F.Z. (grant nos. 2017HWTP2K, 2017L8FWY8 and 2017JWZKP2, respectively) and Bando Ricerca Scientifica di Eccellenza 2018 Fondazione Cariparo grant no. 52008 to G. Brusatin and S.P.
Author information
Authors and Affiliations
Contributions
H.L.S.-M., G. Brumana, A. Guarnieri performed most of the experiments in vitro and in vivo, and contributed to the writing. F.Z. and G. Battilana carried out single-cell analyses and ChIP–PCR. M. Forcato, S.B. and M.C. provided the bioinformatic data. V.G. carried out histology. M. Fassan performed histopathological evaluations. T.P. helped with the mouse colonies. A. Gandin, L.U. and G. Brusatin supported the mechanical bioassays. R.L.X. helped with Remodelin. M. Foiani provided advice and reagents for cGAS. C. Tripodo performed PLA. All authors contributed to the manuscript. S.P. conceived the initial hypothesis and experimental design, organized the work and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Pere Roca-Cusachs, Dennis Discher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Natural aging correlates with loss of YAP/TAZ mechanotransduction in connective tissues.
a, b, Violin plots showing the distribution of the YAP/TAZ activity signature expression in the indicated cell types form young and old mice. Heart fibroblasts (young n = 476 cells; old n = 511 cells), mesenchymal stem cells (young n = 3824 cells; old n = 4249 cells), mammary gland epithelial cells (young n = 873 cells; old n = 1196 cells), bronchiolar epithelial cells (young n = 44 cells; old n = 15 cells), hepatocytes (young n = 2304 cells; old n = 358 cells), distal renal tubule epithelial cells (young n = 225 cells; old n = 147 cells), Henle’s loop epithelial cells (young n = 870 cells; old = 346 cells), Schwann cells (young n = 109 cells; old n = 93 cells), brain neurons (young n = 83 cells; old n = 59 cells), and heart leukocytes (young n = 139 cells; old n = 187 cells) were retrieved from Tabula Muris Senis (as in Fig. 1b). Red lines denote the median, black lines denote quartiles. P values are derived from two-sided, unpaired t-test with Welch’s correction. c, Immunoblotting analysis of YAP and phospho-YAP (p-YAP) in dermal (left panel) and aortic (right panel) samples from young and old mice (n = 3 mice per age and tissue). GAPDH served as loading control. d, f, Representative immunofluorescence staining for phospho-FAK (p-FAK) in dermal fibroblasts (d) and aortic wall (f) of wild-type mice of the indicated ages. elLam is elastic lamellae. Scale bars, 10 µm. e, g, Quantification of the p-FAK signal in dermal fibroblasts (e, related to d) and aortic smooth muscle cells (g, related to f) of wild-type mice of the indicated ages. Data are shown as mean (red line) ± s.d. At least 400 cells per condition were analysed from 3 independent mice. P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. P < 0.0001 in all conditions compared to 3-month-old mice.
Extended Data Fig. 2 YAP/TAZ in dermal fibroblasts control dermal and ECM homeostasis.
a, Representative in situ hybridization for Cre recombinase (CreER) in dorsal skin sections of a Col1a2-CreER mouse, showing specific staining in dermal fibroblasts (Scale bar, 50 µm). b, Quantification of Vimentin-positive cells in the dermal layer of skin sections from mice of the indicated genotypes. Data (n = 4 mice for each column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. Related to Fig. 2b. c, Quantification of Cdkn1a-positive cells in the dermal layer of skin sections from mice of the indicated genotypes. Data (n = 4 per condition) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. Related to Fig. 2c. d, e, Representative birefringence images (d, Scale bar, 100 µm) and quantification (e) of picrosirius red staining on dorsal skin sections from mice of the indicated genotypes. In d, the red-to-green gradient reflects the decreasing thickness of collagen fiber bundles. In e, red is the quantification of thicker collagen fibers appearing as red or yellow in birefringence; green is the quantification of thinner fibers appearing as green in the same conditions. Data in e (n = 4 mice for each column) are presented as mean + s.d., 25 areas were analyzed per condition. f, Violin plots from sc-RNA seq data showing the expression distribution of Cdkn1a and Btg2 in freshly dissociated dermal fibroblasts of young control (n = 2916 cells), young Y/T cKO (n = 759 cells) and old control (n = 1413 cells) mice. Red lines denote the median, black lines denote quartiles. P values are derived from Kruskal-Wallis test. g, Immunoblotting analysis of YAP in tail tip biopsies from wild-type (WT Control) and R26-rtTAM2; Col-tetO-YAPS127A mice treated with intermittent doxycycline regime to sustain YAP activity. Mice were analyzed under doxycycline (Doxy Pulse) or during the off-doxy period, two or four days after the last doxy pulse (Wash out d2 and Wash out d4, respectively; see Methods). GAPDH served as loading control.
Extended Data Fig. 3 Additional characterization of the role of YAP/TAZ mechanotransduction in preserving aortic wall integrity.
a, Whole mount X-Gal staining in heart and aorta (upper panel) and aortic wall sections (lower panel, Scale bars, 50 µm) of SmmhcCreER; R26-LSL-LacZ mice, either treated with Tamoxifen or Vehicle. Blue signal confirms the inducible and highly specific activity of the Cre driver in vascular smooth muscle cells. b, Representative immunofluorescence staining for alpha-smooth muscle actin (αSMA, upper panel) and YAP/TAZ (lower panel) in the aortic wall of SmmhcCreER; YAPfl/fl; TAZfl/fl mice, showing YAP/TAZ ablation in vascular smooth muscle cells exclusively after Tamoxifen treatment. Scale bar, 10 µm. c, Quantification of medial layer thickness, adventitial layer thickness and elLam fragmentation of mice of the indicated genotypes. Young control are 7-month-old wild-type mice, Old Control are > 21-month-old wild-type mice. Related to Fig. 3a. Data (n = 8 mice for each column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. d, e, Representative immunofluorescence staining for p-MLC2 (d, Scale bar, 10 µm) and quantification (e) of p-MLC2 levels in the aortic wall of wild-type (WT) and Fbn1C1039G/WT mice of the indicated ages. elLam is elastic lamellae. Quantification data are presented as mean ± s.d. P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. P < 0.0001 in all conditions compared to 3-month-old wild-type mice, except for 3-month-old Fbn1C1039G/WT mice (P = 0.0092). The number of cells analyzed for each mouse strain and age is reported in the corresponding source data file (where n is > 400 for each point). f, Immunoblotting analysis of total FAK and phospho-FAK (p-FAK) in adventitia-denuded aortic samples from wild-type and 7-month-old Fbn1C1039G/WT mice. α-Tubulin (αTUB) served as loading control. Two mice per condition are shown. g, Quantification of medial layer thickness and adventitial layer thickness in aortic sections of wild-type, Fbn1C1039G/WT and Fbn1C1039G/WT + YAP mice, as in Fig. 3f. Data (n = 8 mice for each group) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test.
Extended Data Fig. 4 YAP/TAZ oppose senescence in stromal cells.
a, b, Heatmaps showing transcriptional induction (a) and secretion (b) of SASP factors after ex vivo genetic ablation of YAP/TAZ (Y/T cKO) in primary mouse adult fibroblasts (MAFs). Secretion of TNFα, a necrosis marker, was not detected. c, Heatmaps showing upregulation of SASP in WI-38 human fibroblasts upon siRNA-mediated depletion of YAP/TAZ (siY/T). TNFA expression was not detected. d, RT–qPCR analysis showing induction of SASP marker genes in adventitia-denuded aortic mediae from SMC-Y/T cKO compared to control mice. Data (n = 8 mice per column) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. e, RT-qPCR analysis of SASP marker genes in control (siCo) and YAP/TAZ-depleted (siY/T) WI-38 cells, treated with CDK4 inhibitor (CDK4i, 4 μM Palbociclib for 48 h), with or without concomitant STING inhibition (STINGi, 1 µM H-151 for 48 h). Data (n = 3 independent experiments) are shown as mean ± s.d. f, Senescence-associated β-galactosidase (SA-β-Gal) staining (left panel, Scale bar, 50 µm) and quantification (right panel, n = 3 independent experiments) in WI-38 cells upon depletion of YAP/TAZ (siY/T). Quantification data are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. g, Heatmap showing upregulation of SASP marker genes in primary old MAFs, rescue by YAP-add back, as assessed by RT-qPCR. MAFs were isolated from young and old R26-rtTAM2; Col-tetO-YAPS127A mice, left untreated (Control) or treated with doxycycline (+ YAP) to sustain YAP activity ex vivo. Related to Fig. 4b. h, SA-β-Gal staining (left panel, bar = 50 µm) and quantification (right panel, n = 3 independent experiments) in primary MAFs isolated from young (3 months) and old (>21 months) mice, in the presence or absence of constitutively active YAP5SA (+ YAP). Cells transduced with empty vector are indicated as Control. Quantification data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. i, Immunofluorescence staining for YAP/TAZ (Y/T) (left panel, Scale bar, 10 µm) and quantification of subcellular Y/T distribution (right panel, n = 3 independent experiments) in freshly isolated MAFs from old (>21 months) mice, either control or treated with Pyrintegrin (3.5 μM for 24 h). Data are displayed as mean ± s.d. j, RT-qPCR analysis of Cxcl10 in primary MAFs isolated from young (3 months) and old (>21 months) mice, the latter treated with either vehicle (control) or Pyrintegrin (3.5 μM for 24 h). Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. k, Immunofluorescence staining for YAP/TAZ (Y/T) (left panel, Scale bar, 10 µm) and quantification of subcellular Y/T distribution (right panel, n = 3 independent experiments) in freshly isolated MAFs from young mice, either control (vehicle) or treated with Rho inhibitor (Rhoi, 0.5 μg/ml C3 for 24 h). Quantification data are displayed as mean ± s.d. l, Expression of SASP markers in young MAFs either control (vehicle) or treated with Rhoi, as assessed by RT-qPCR. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test.
Extended Data Fig. 5 YAP/TAZ loss triggers cGAS activation on genomic DNA.
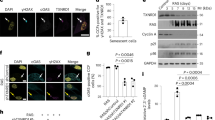
a, cGAMP levels in control (siCo) or YAP/TAZ-depleted (siY/T) primary mouse aortic smooth muscle cells, as assessed by ELISA. Concomitant cGAS depletion (sicGAS #1 and #2) served as specificity control. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. b, Immunofluorescence staining (left panel, Scale bar, 20 µm) and quantification (right panel, n = 3 independent experiments) for endogenous cGAS in freshly isolated control (siCo) and YAP/TAZ-depleted (siY/T) MAFs. Concomitant cGAS depletion (siY/T + sicGas) served as specificity control. siY/T middle panel is shown as low magnification, field view of the same cells shown at high magnification in Fig. 4d. Data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. c, Confocal images (left panel, Scale bar, 10 µm), superresolution microscopy analysis (middle panel, Scale bar, 1 µm. Arrowheads indicate sites of genomic DNA herniation through the nuclear Lamina (Lamin A/C)) and quantification (right panel, n = 3 independent experiments) of EGFP-cGAS reporter signal in control (siCo) and YAP/TAZ-depleted (siY/T) WI-38 cells. Quantification data are shown as mean ± s.d; P values are derived from two-sided, unpaired t-test. d, 3D reconstruction of immunofluorescence-labelled (Lamin A/C) nuclei of YAP/TAZ-depleted (siY/T) WI-38 cells, expressing the EGFP-cGAS reporter. Arrowheads indicate EGFP-cGAS recruitment at sites of genomic DNA (DAPI) herniations. Scale bar, 1 μm. e, Low magnification(field view) of endogenous cGAS IHC staining in the dermis of control and Y/T cKO mice (left panel, Scale bar, 10 µm). Quantifications (right panel, n = 5 mice) are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test. f, Quantification of cGAS-positive aortic smooth muscle cells from control and SMC-Y/T cKO mice. Data (n = 4 mice per column) are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test. Related to Fig. 4g. g, IHC staining (left panel, Scale bar, 10 µm) and quantification (right panel, n = 4 mice per column) of endogenous cGAS protein in kidney glomeruli of control and Y/T cKO mice. Quantification data are shown as mean ± s.d; P values are derived from two-sided, unpaired t-test. h, In vivo PLA detection (arrowheads, left panel, Scale bar, 1 µm) of physical association of endogenous cGAS with nuclear DNA in dermal fibroblasts of control and YAP/TAZ cKO mice (respectively, 2% and 19% of PLA-positive cells, n > 30 cells). Single antibodies (α-cGAS and α-DNA) run in the same assay gave no signal whatsoever, and representative pictures of these negative controls are shown in the right panel.
Extended Data Fig. 6 YAP/TAZ mechanotransduction suppresses cGAS activation.
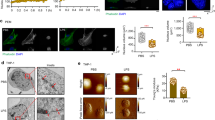
a, EGFP-cGAS reporter signal (left panel, Scale bar, 10 μm) and quantification (right panel, n = 3 per condition) in WI-38 cells seeded on stiff (40 kPa) and soft (0.5 kPa) hydrogels in presence or absence of experimentally sustained YAP activity (+YAP). Cell boundaries are indicated by dotted lines. Quantification data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. b, 3D reconstruction of a representative nucleus from WI-38 cells expressing the EGFP-cGAS reporter seeded on soft (0.5 kPa) hydrogels and then stained with Lamin A/C and DAPI. Arrowheads indicate EGFP-cGAS accrual at site of genomic DNA (DAPI) herniation. Scale bar, 2 µm. c, EGFP-cGAS reporter signal (left panel, Scale bar, 10 μm) and quantification (right panel, n = 3 independent experiments) in WI-38 cells plated on large and small adhesive microislands. Cell boundaries are indicated by dotted lines. Quantification data are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. d, Representative immunofluorescence staining (left panel, Scale bar, 10 µm) and quantification (right panel, n = 4 mice) of endogenous cGAS in the aortic wall of mice of the indicated experimental groups. elLam is elastic lamellae. Note perinuclear activation of endogenous cGAS in the aortic mediae of Fbn1C1039G/WT mice, and its prevention by sustaining YAP activity (Fbn1C1039G/WT+YAP) in aortic smooth muscle cells. Data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. e, RT-qPCR analysis of senescence markers in adventitia-denuded aortic mediae from mice of the indicated genotypes. Data (n = 8 mice per column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test.
Extended Data Fig. 7 YAP/TAZ loss induces SASP induction through cGAS/STING signalling.
a–c, Heatmap showing the STING-dependent induction of inflammatory genes (a), interferon target genes (b) and NF-kB target genes (c) upon YAP/TAZ depletion in WI-38 fibroblasts, as determined by RNA-seq. d, Heatmap showing that E2F target genes are downregulated upon YAP/TAZ depletion in WI-38 cells independently of STING, as determined by RNA-seq. e, Heatmap portraying the STING-signaling dependency of SASP marker induction upon genetic ablation of YAP/TAZ (Y/T cKO) in primary MAFs. STINGi is H-151 (1 µM). f, Heatmap displaying cGAS-dependent cytokine secretion upon genetic ablation of YAP/TAZ in primary MAFs. g, RT-qPCR for SASP marker genes upon YAP/TAZ depletion (siY/T) in mouse aortic smooth muscle cells, with or without concomitant cGAS (sicGas#1 and #2) or STING (siSting#1 and #2) depletion. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. h, Cytokine array showing cGAS-dependent secretion of the indicated SASP cytokines upon YAP/TAZ ablation in primary mouse aortic smooth muscle cells. Spots in the upper left and right corners and in the lower right corner served as reference. i, In situ hybridization for CXCL10 in control (siCo) and YAP/TAZ-depleted (siY/T) WI-38 cells, expressing EGFP-cGAS reporter. Treatment with STING inhibitor (STINGi, 1 µM H-151 for 48 h) served as negative control. Scale bar, 2 μm. Percentage of cells showing concomitant EGFP-cGAS activation and CXCL10 mRNA expression were 0% in siCo, 35% in siY/T and 0% in siY/T + STINGi conditions. At least 40 cells were analyzed for each condition. j, Upper panel, RT-qPCR for SASP marker genes in YAP/TAZ-depleted (siY/T) and control (siCo) WI-38 cells, treated with 2',3'-dideoxycytidine (ddC, 5 µM for 1 week). Lower panel, qPCR for mitochondrial genes confirming mtDNA depletion by ddC treatment. Note that mtDNA is not required for SASP induction after Y/T depletion. Data (n = 3 independent experiments) are presented as mean ± s.d.
Extended Data Fig. 8 cGAS-STING signalling drives senescence-traits and age-related pathology downstream of YAP/TAZ inactivation in connective tissues.
a, Representative ISH for Mmp3 (Scale bar,10 µm) in the dermis from mice of the indicated genotypes. b, c, Quantification (n = 5 mice per column) of the ISH signal for the indicated SASP markers in the dermis (b, related to a, and to Fig. 4i) and aorta (c, related to Fig. 4j) from mice of the indicated genotypes. Data are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. d, RT-qPCR analysis showing induction of SASP marker genes in aortic mediae of SMC-Y/T cKO mice, prevented by STING loss-of-function mutation (SMC-Y/T cKO; STINGGt/Gt). Data (n = 8 mice per column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. e, f, Representative ISH for Il1b (e, Scale bars, 10 µm) and quantification (f, n = 5 mice) of the ISH signal for the indicated SASP markers in kidney sections from young control, Y/T cKO and Y/T cKO; STINGGt/Gt mice. Quantification data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. Related to Fig. 4k. g, Quantification of Vimentin-positive cells (n = 4 mice per column), subcutaneous fat layer thickness (n = 3 mice) and hair follicle density (n = 4 mice) in skin sections of mice of the indicated genotypes. Related to Fig. 4l. Data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. h, Quantification of medial layer thickness, adventitial layer thickness and elastic lamellae fragmentation of aortic sections from mice of the indicated genotypes. Related to Fig. 4m. Data (n = 5 mice per column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test.i, j, Representative elLam staining (i, Scale bar, 100 µm) and quantification (j, n = 4 mice per column) of medial layer thickness, adventitial layer thickness and elLam fragmentation in aortic sections from control mice, and SMC-Y/T cKO mice either treated with Vehicle or with the STING inhibitor C-176. Data are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. k, l, Representative images of H&E-stained (k, Scale bar, 100 µm) kidney sections of mice of the indicated genotype. Quantification (l) of intratubular casts (n = 5 mice per column), dilated tubules (n = 6 mice per column) shown in k, and sclerotic glomeruli shown in Fig. 4n (n = 5 for mice per column) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. m, Quantification of γH2AX signal in dermal fibroblasts of control and YAP/TAZ cKO mice. The number of cells analyzed for each condition is reported in the corresponding source data file (n > 150 cells). Data are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test.
Extended Data Fig. 9 YAP/TAZ mechanotransduction is required to preserve NE architecture.
a, Quantification of nuclear dysmorphia (related to Fig. 5a) in young control or YAP/TAZ-depleted MAFs, and in old MAFs with or without YAP reconstitution. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. b, Nuclear morphology (left panel) and quantification of nuclear dysmophia (right panel) in control (siCo) and YAP/TAZ-depleted (siY/T) WI-38 cells, as assessed by Lamin A/C immunostaining. Scale bar, 10 μm. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. c, Orthogonal projection of Lamin A/C-stained nuclei (left panel, Scale bar, 1 µm) and quantification of nuclear height (right panel; n = 70 cells per condition) in control or YAP/TAZ-depleted WI-38. Data are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. d, Pie charts showing the relative percentage of nuclear lamina defects upon Y/T depletion in WI-38 fibroblasts. At least 200 cells per condition were analyzed. e, f Nuclear morphology (right panel) and quantification (left panels) of nuclear dysmorphia in WI-38 cells plated on stiff (40 kPa) or soft (0.5 kPa) hydrogels (e) and WI-38 cells plated on large or small microislands (f), as assessed by Lamin A/C immunostaining. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. Scale bar, 10 μm. g, Nuclear morphology (left panel, Scale bar, 10 μm) and quantification (left panel) of nuclear dysmorphia in YAP/TAZ-depleted WI-38 cells, in presence or absence of Remodelin, as assessed by Lamin A/C immunostaining. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. h, Quantification of EGFP-cGAS reporter signal in YAP/TAZ-depleted (siY/T) WI-38 cells, with or without Remodelin treatment. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. Related to Fig. 5b. i, j SASP marker genes expression in WI-38 cells (i, n = 2 independent experiments) and primary MAFs (j, n = 3 independent experiments) upon YAP/TAZ depletion is abolished by concomitant Remodelin (Rem) treatment, as assessed by RT-qPCR analysis. In j, treatment with the STING inhibitor H-151 (STINGi) served as control. Data are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. k, In vivo super-resolution microscopy analysis (left panel, Scale bar, 1 μm) and quantification (right panel, at least n = 130 cells per condition were analyzed) of perinuclear F-Actin in dermal fibroblasts of Control and YAP/TAZ cKO mice. Data are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test.
Extended Data Fig. 10 Role of the actin cap in preserving nuclear morphology downstream of YAP/TAZ.
a, Quantification of actin cap integrity (related to Fig. 5c) in young control or YAP/TAZ-depleted MAFs, and in old MAFs with or without YAP reconstitution. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. b, Superresolution microscopy analysis (left panels) of actin cap integrity in YAP/TAZ-depleted WI-38 cells, in presence or absence of Remodelin, as assessed by F-Actin and Lamin A/C staining. Apical sections are shown. Scale bar, 10 μm. Quantifications (right panel; n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. c, Nuclear morphology in WI-38 cells expressing dominant-negative KASH1 domain (KASH1DN) or depleted of Nesprin1/2 (siNespr1/2), as assessed by Lamin A/C immunostaining. Scale bar, 10 μm. d, Quantification (related to c and Fig. 5d) of nuclear dysmorphia and EGFP-cGAS (perinuclear or nuclear accrual) upon disruption of the LINC complex. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. e, Heatmap displaying SASP induction in WI-38 cells expressing dominant negative KASH1 domain (KASH1DN), with or without concomitant STING inhibition (STINGi, 1 μM H-151), as assessed by RT-qPCR. f, Heatmap displaying SASP induction in LINC-disrupted (siNespr1/2, siSUN1/2) WI-38 cells, as assessed by RT-qPCR. g, Actin cap disruption (quantification in right panel) coinciding with NE dysmorphia in Nesprin1/2-depleted cells, as visualized by F-Actin and Lamin A/C staining. Scale bar, 10 μm. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. h, Left: RT-qPCR analysis of LMNB1 expression levels in control (siCo) and YAP/TAZ-depleted (siY/T #1 and #2) WI-38 cells. Right: Immunoblot analysis of Lamin B1 protein levels in WI-38 cells and primary adult fibroblasts (MAFs) upon YAP/TAZ depletion. i, Heatmap showing cGAS-dependent SASP induction upon Lamin B1 depletion (siLMNB1) in WI-38 cells, as assessed by RT-qPCR. j, RT-qPCR analysis of SASP marker genes in WI-38 cells upon Lamin B1 (siLMNB1 #1 and #2) or YAP/TAZ (siY/T) depletion. k, RT-qPCR analysis of ACTR2 expression levels in control (siCo) and YAP/TAZ-depleted (siY/T #1 and #2) WI-38 cells. In h, j, kdata (n = 3 independent experiments) are presented as mean ± s.d. P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. l, Quantification of actin cap integrity, nuclear dysmorphia and perinuclear EGFP-cGAS accrual upon ACTR2 depletion (siACTR2) in WI-38 cells, 48 h after transfection. Related to Fig. 5e. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. m, Quantification (left) of mean focal adhesion (FA) length (n = 3 independent experiments) and abundance (n = 25 cells per condition) upon ACTR2 knockdown in WI-38 cells, 48 h after siRNA transfection. Data are shown as mean ± s.d.; P values are derived from two-sided, unpaired t-test. On the right, F-Actin and Vinculin staining (bar = 10 μm) in control and ACTR2 -depleted WI-38 cells. Apical and basal sections are shown. Cells were fixed for IF at 32 h after siRNA transfection. n, cGAMP levels in control (siCo) and ACTR2-depleted (siACTR2) WI-38 cells, as assessed by ELISA. Concomitant cGAS depletion served as specificity control. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. o, Heatmap showing cGAS-dependent SASP induction upon ACTR2 depletion (siACTR2) in WI-38 cells, as assessed by RT-qPCR. p, EGFP-cGAS reporter signal (left panel) and quantification (right panel, n = 3 independent experiments) in WI-38 cells either control or treated with ARP2/3 inhibitor (ARP2/3i, 50 μM CK-869 for 12 h). Scale bar, 10 μm. Data are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. q, RT-qPCR analysis of SASP marker genes in WI-38 cells either control or treated with the ARP2/3 inhibitor (ARP2/3i) CK-869 at the indicated doses. Data (n = 3 independent experiments) are presented as mean ± s.d. r, Quantification of WI-38 cells displaying intact actin cap, nuclear dysmorphia and perinuclear EGFP-cGAS accrual upon YAP/TAZ depletion, with or without concomitant ARPIN depletion. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. Related to Fig. 5e.
Extended Data Fig. 11 Low mechanics disrupt the nuclear envelope and activate cGAS/STING by inhibiting YAP/TAZ.
a, RT-qPCR analysis (left panel) of Lmnb1 and ACTR2 in MAFs and WI-38 cells, respectively, plated on stiff (40 kPa) or soft (0.5 kPa) hydrogels. Lmnb1 and ACTR2 expression on soft substrate is rescued by add-back of constitutively active YAP. Of note, cell cycle inhibition (CDK4/6i treatment, 4 μM Palbociclib for 48 h) in WI-38 cells plated on stiff substrates is inconsequential for LMNB1 and ACTR2 expression levels (right panel). Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test (left panel) and from two-sided, unpaired t-test (right panel). b, Superresolution microscopy analysis (left panel) of actin cap integrity, as visualized by F-Actin and Lamin A/C staining, and quantification (right panel) in WI-38 cells plated on adhesive microislands of the indicated sizes. Scale bar, 10 μm. Data (n = 3 independent experiments) are presented as mean ± s.d.; P values are derived from two-sided, unpaired t-test. c, RT-qPCR analysis of YAP/TAZ target genes and SASP marker genes in WI-38 cells plated on stiff (40 kPa) or soft (0.5 kPa) hydrogels in the presence (+YAP) or absence (Control) of constitutively active YAP5SA. Control cells were transduced with empty vector. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. d, RT-qPCR analysis of YAP/TAZ target genes and SASP marker genes in primary MAFs plated on stiff (40 kPa) or soft (0.5 kPa) hydrogels. MAFs were isolated from R26-rtTAM2; Col-tetO-YAPS127A mice and either left untreated (Control) or treated with doxycycline (+ YAP) ex vivo to sustain YAP activity. Data (n = 3 independent experiments) are shown as mean ± s.d.; P values are derived from one-way ANOVA with Dunnett’s multiple comparison test. e, ChIP-Seq profiles for YAP in human NCI-H2052 cells and human IMR90 fibroblasts showing YAP binding at enhancer elements mapped (red arrow) to the ACTR2 promoter, according to published Hi-C interactome maps. f, Independent repeat experiment of ChIP-qPCR shown in Fig. 5i. Enrichment relative to siY/T: 25.4-fold for TAZ-IP on LMNB1 promoter, 13.8-fold for IP-YAP on LMNB1 promoter; 62.7-fold for IP-TAZ on ACTR2 enhancer, and 99-fold for IP-YAP on ACTR2 enhancer. The panel shows the result of one experiment. g, ChIP-qPCR analysis in human vascular smooth muscle cells (vSMCs) showing that the regulatory elements for LMNB1 and ACTR2 are enriched in YAP- and TAZ-immunoprecipitated chromatin, but not in negative control IP (IgG). Relative DNA binding was calculated as fraction of input and normalized to IgG. The panel shows the result of one experiment, independent repeats for a different cell line (human WI-38) are shown in Fig. 5i and Extended Data Fig. 11f. h, YAP/TAZ-depletion in primary MAFs is inconsequential for Lmna expression levels, as assessed by RNA-Seq. Results of two independent experiments are shown.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 and legends for Supplementary Tables.
Source data
Rights and permissions
About this article
Cite this article
Sladitschek-Martens, H.L., Guarnieri, A., Brumana, G. et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS–STING. Nature 607, 790–798 (2022). https://doi.org/10.1038/s41586-022-04924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04924-6
This article is cited by
-
Noggin promotes osteogenesis in human adipose-derived mesenchymal stem cells via FGFR2/Src/Akt and ERK signaling pathway
Scientific Reports (2024)
-
Longevity interventions modulate mechanotransduction and extracellular matrix homeostasis in C. elegans
Nature Communications (2024)
-
Cytosolic DNA sensors in neurodegenerative diseases: from physiological defenders to pathological culprits
EMBO Molecular Medicine (2024)
-
Distinguishable DNA methylation defines a cardiac-specific epigenetic clock
Clinical Epigenetics (2023)
-
Divergent roles of the Hippo pathway in the pathogenesis of idiopathic pulmonary fibrosis: tissue homeostasis and fibrosis
Inflammation and Regeneration (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.