Abstract
Broadly neutralizing antibodies that target epitopes of haemagglutinin on the influenza virus have the potential to provide near universal protection against influenza virus infection1. However, viral mutants that escape broadly neutralizing antibodies have been reported2,3. The identification of broadly neutralizing antibody classes that can neutralize viral escape mutants is critical for universal influenza virus vaccine design. Here we report a distinct class of broadly neutralizing antibodies that target a discrete membrane-proximal anchor epitope of the haemagglutinin stalk domain. Anchor epitope-targeting antibodies are broadly neutralizing across H1 viruses and can cross-react with H2 and H5 viruses that are a pandemic threat. Antibodies that target this anchor epitope utilize a highly restricted repertoire, which encodes two public binding motifs that make extensive contacts with conserved residues in the fusion peptide. Moreover, anchor epitope-targeting B cells are common in the human memory B cell repertoire and were recalled in humans by an oil-in-water adjuvanted chimeric haemagglutinin vaccine4,5, which is a potential universal influenza virus vaccine. To maximize protection against seasonal and pandemic influenza viruses, vaccines should aim to boost this previously untapped source of broadly neutralizing antibodies that are widespread in the human memory B cell pool.
Similar content being viewed by others
Main
Antibodies to the major surface glycoprotein haemagglutinin (HA) are critical for providing protection against influenza virus infection6,7. However, most HA-binding antibodies target variable epitopes of the HA head domain, which provide limited protection against antigenically similar influenza virus strains3. Vaccine formulations that preferentially induce antibodies to conserved epitopes of the HA head and stalk domains could provide broad and potent protection against a wide array of influenza viruses. Several leading universal influenza virus candidates are designed to induce antibodies specifically to the stalk domain, but the spectrum of distinct epitopes on the stalk targeted by the human B cell repertoire remains to be determined. By analysing the specificities of B cells targeting the H1 stalk through the generation of monoclonal antibodies (mAbs), our study reveals a new class of broadly neutralizing antibodies (bnAbs) to an underappreciated epitope where HA anchors itself into the viral membrane. Next-generation vaccine platforms should take advantage of this finding to elicit antibodies to the conserved anchor epitope.
Discovery of anchor epitope-binding mAbs
To investigate the specificities of HA-specific antibodies, we generated 358 mAbs from plasmablasts and HA+ memory B cells (MBCs) isolated from volunteers who were vaccinated against or naturally infected with seasonal influenza viruses or were participants in a phase I clinical trial of a chimeric HA (cHA) vaccine4,5. Of all mAbs tested, nearly half targeted the HA stalk domain, 21% of which targeted the well-characterized central stalk (CS) epitope (Extended Data Fig. 1a). Notably, stalk-binding mAbs were disproportionally isolated from the infected, 2009 pandemic H1N1 (pH1N1) monovalent inactivated influenza vaccine, and the cHA vaccine cohorts (Extended Data Fig. 1b), as these exposure routes have previously been shown to induce antibody responses to the HA stalk5,8,9. To investigate which epitopes the remaining 79% of stalk-binding mAbs were targeting, we performed negative-stain electron microscopy with three non-CR9114 (ref. 10) competing stalk domain-binding mAbs: 047-09 4F04, 241 IgA 2F04 and 222-1C06. All three mAbs bound an epitope near the anchor of the HA stalk and were oriented at an upward angle towards the epitope (Fig. 1a, b, Extended Data Fig. 1c), suggesting that this epitope may be partially obstructed by the lipid membrane and may only be exposed for antibody binding as the HA trimers flex on the membrane. FISW84, a recently identified stalk-binding mAb11, targets an epitope that overlaps with the three identified anchor-binding mAbs (Extended Data Fig. 1d), suggesting that the anchor epitope is a common stalk epitope. Moreover, a proximal epitope was previously identified on group 2 viruses that is targeted by mAbs CR8020 (ref. 12) and CR8043 (ref. 13). Despite some overlap, the epitope targeted by the group 2 mAbs was considerably farther up and to the right on the HA stalk relative to the anchor epitope, and mAbs CR8020 and CR8043 targeted the stalk from above (Extended Data Fig. 1e, f), at an angle and positioning more akin to antibodies targeting the CS epitope. mAbs binding the CS epitope (CR9114 (ref. 10) and FI6v3 (ref. 14)) did not have overlapping footprints or compete for HA binding with the anchor-binding 047-09 4F04 mAb in an HA competition assay (Fig. 1c, d). In total, we identified 50 distinct mAbs that competed for binding to the anchor epitope from a total of 21 individuals (Fig. 1d, Extended Data Tables 1, 2). Of these, 34 anchor-binding mAbs from 15 donors were isolated from the cHA vaccine trial (Extended Data Tables 1, 2).
a, Negative-stain EM of representative 2D class averages and 3D reconstructions of Fabs binding to A/California/7/2009 (E376K) HA. 047-09 4F04 was imaged at ×52,000 normal magnification and 222-1C06 and 241 IgA 2F04 were imaged at ×62,000 normal magnification. b, Juxtaposed 3D reconstructions of Fabs binding to A/California/7/2009 (E376K) HA. c, Binding footprints of anchor-binding Fabs relative to mAbs targeting the CS epitope (CR9114 and FI6v3). d, Competition of stalk-binding mAbs with CR9114 or 047-09 4F04 (bold mAbs). e, Neutralization potency of anchor-binding (n = 50) and CS-binding (n = 37) mAbs to A/California/7/2009. Data are represented as mean ± s.d. and were analysed by a two-tailed unpaired non-parametric Mann–Whitney test. IC50, half-maximal inhibitory concentration. f, Proportion of anchor-targeting (n = 50) and CS-targeting (n = 37) mAbs binding to other group 1 influenza virus A subtypes. Data were analysed by Fisher’s exact tests. g, Representative 2D class averages (×62,000 normal magnification), 3D reconstructions and footprints of 222-1C06 binding to H2 and the relative footprint on H1. See also Extended Data Figs. 1–3.
Anchor-binding mAbs were broadly reactive and broadly neutralizing to pre-pandemic and post-pandemic H1N1 viruses and a swine-origin H1N2 virus (Fig. 1e, Extended Data Fig. 1g, h). Notably, anchor-binding mAbs had similar neutralizing potency to pH1N1 relative to mAbs to the CS epitope (Fig. 1e, Extended Data Fig. 1i, j). Many stalk-targeting antibodies mediate protection via Fc-mediated functions, including antibody-dependent cellular cytotoxicity15,16. Anchor epitope-binding mAbs largely did not possess antibody-dependent cellular cytotoxicity activity (15 out of 18; Extended Data Fig. 1k–l), potentially due to the upward angle of approach of anchor-binding antibodies, which may position the Fc distantly from effector cells. Despite pan-H1 binding, anchor-targeting mAbs rarely cross-reacted with other HA subtypes tested, including H3, a group 2 subtype, other group 1 subtypes, including H2 and H5, and influenza B viruses (Fig. 1f, Extended Data Fig. 2a). Despite this, the 222-1C06 mAb cross-reacted with H2 and H5 HA (Fig. 1g, Extended Data Fig. 2b) and several anchor-binding antibodies could neutralize an H2N2 virus (Extended Data Fig. 2c), suggesting that antibodies targeting the anchor epitope can cross-neutralize other group 1 influenza A viruses. We recently demonstrated that bnAbs to the HA stalk are often polyreactive17, which may limit the activation of B cells expressing bnAbs. Relative to mAbs that target the CS epitope, we identified that anchor-binding mAbs were proportionally less likely to be polyreactive and those that were polyreactive had weaker relative affinity for lipopolysaccharide (Extended Data Fig. 2d, e). These data suggest that, although polyreactivity is selected for within the anti-anchor epitope B cell pool, it is not to the same level as B cells to the CS epitope.
H1N1 viruses have acquired several mutations within the HA stalk domain, probably due to antibody selective pressures or to increase stability. To understand whether these mutations affect antibody binding to the anchor epitope, we screened mAbs against naturally occurring and experimentally identified viral escape mutants of mAbs binding to the CS epitope (Extended Data Fig. 2f, g, Extended Data Table 3). Anchor epitope-binding mAbs were mostly unaffected by these mutants, whereas most of the CS-binding mAbs showed reduced binding to at least one mutant (Extended Data Fig. 2g). Notably, most mAbs had reduced binding to A373V of HA2, which has recently been shown to preferentially grow in the presence of mAbs to the CS epitope2. While A373 is distant from the anchor epitope, the A373V mutation has been shown to affect the conformation of the HA stalk2, explaining the broad reduction of HA binding by antibodies targeting either stalk epitope. Anchor-binding mAbs only demonstrated a 10–30% reduction in binding (Extended Data Fig. 2g), indicating that they are still likely to neutralize viruses carrying the A373V mutation.
To test whether mAbs targeting the anchor epitope were protective in vivo, we administered a cocktail of five mAbs targeting the anchor epitope or the CS epitope prophylactically and therapeutically (48 h after infection) to mimic a polyclonal response against these epitopes and infected mice with a lethal dose of a mouse-adapted pH1N1 virus (A/Netherlands/602/2009; Supplementary Table 1). Mice that received a prophylactic or therapeutic anti-anchor cocktail at 5 mg/kg experienced 100% protection from weight loss and lethal infection (Extended Data Fig. 3a, b). No differences in lung viral titres were detected in mice that received the anti-anchor cocktail prophylactically relative to mice administered the negative control mAb (Extended Data Fig. 3c). Anti-stalk antibodies do not provide sterilizing immunity but are known to neutralize subsequent rounds and limit disease progression18. As a result, lung viral titres do not necessarily reflect protection from morbidity and mortality. Finally, the anti-anchor cocktail given prophylactically provided 70% protection against lethal A/Fort Monmouth/1/1947 infection (Extended Data Fig. 3d), a virus that circulated before the birth of any of the donors in our study (Extended Data Table 1). Since anchor-binding mAbs do not engage in antibody-dependent cellular cytotoxicity for the most part, antibodies that target the anchor epitope probably provide protection in vivo through direct neutralization of the virus. Together, these data indicate that antibodies to the anchor epitope are pan-H1 neutralizing and protective in vivo.
Structure of an anchor-binding antibody
All anchor epitope-binding mAbs utilized one of four VH3 genes: VH3-23, VH3-30 or VH3-30-3, and VH3-48, in contrast to mAbs targeting the CS epitope, which commonly use VH1-69 (Fig. 2a, Extended Data Fig. 4a, b). Anchor epitope-binding mAbs also utilized a highly restricted light chain repertoire, with all mAbs utilizing a combination of VK3-11 or VK3-15 combined with JK4 or JK5 (Fig. 2b, Extended Data Fig. 4c, d). Furthermore, all but one light chain of the anchor-targeting mAbs were clonally related (Extended Data Table 2), indicating that the light chains were very similar across mAbs and study participants. We identified four distinct clonal expansions, with one public clone found across two donors (Extended Data Fig. 4e, f, Extended Data Table 2). Anchor epitope-targeting and CS-targeting mAbs exhibited similar levels of somatic hypermutations (Extended Data Fig. 4g). The κ-chain complementarity-determining region 3 (K-CDR3) length of anchor epitope-binding mAbs was highly restricted, with all K-CDR3s being ten amino acids long (Extended Data Fig. 4h).
a, b, VH (a) and VK (b) gene usage by anchor-binding mAbs. The number in the centre of the pie graphs indicates the number of mAbs analysed. c, Cryo-EM structure of anchor-targeting 222-1C06 (blue) and lateral patch-targeting 045-09 2B05 (dark grey; see Methods) binding to A/California/7/2009 (E376K) HA (light grey). d, Heavy chain and light chain footprint of 222-1C06 binding to HA based on the cryo-EM structure. e, HA epitope contact residues (maroon) and heavy chain (green) and light chain (yellow) antibody contact residues of the 222-1C06 paratope. Peach-highlighted amino acids represent the fusion peptide of HA2. f, K-CDR3 NWP and H-CDR2 Y58 motifs of 222-1C06. Bold residues are HA residues. g, Major contacts of 222-1C06 K-CDR1 and H-CDR3 (normal typeface) binding to HA (bold residues). h, i, Weblogo plot and germline sequence of Y58 following the H-CDR2 motif (h) and the K-CDR3 NWP motif (i). j, k, Amino acid conservation of s contact residues across human, swine and avian H1 viruses (j) and group 1 influenza A viruses (k). Bold residues are contacts conserved with A/California/7/2009 H1N1 (k). The strain information used for conservation models in j and k are in Supplementary Tables 4, 5, respectively. See also Extended Data Fig. 4.
To investigate the binding motif of anchor-targeting mAbs, we generated a high-resolution (3.38 Å) cryo-electron microscopy (cryo-EM) structure of 222-1C06 bound to A/California/7/2009 HA (Fig. 2c, Extended Data Fig. 4i–k). The broad paratope of 222-1C06 made extensive contacts across the HA fusion peptide19 (Fig. 2d, e), which mediates viral membrane fusion with the host membrane. Binding of the fusion peptide was largely mediated by an NWP motif within the K-CDR3, a Y58 directly following the H-CDR2, and a W99 in the H-CDR3, with these HA-binding motifs acting independently and in combination via an aromatic pocket (Fig. 2f, g, Supplementary Table 2). Moreover, both the K-CDR3 NWP and the H-CDR2 Y58 were found in all the anchor-binding mAbs and were germline encoded (Fig. 2h, i), which could have led to the selection of B cells utilizing these variable genes. Notably, FISW84 utilizes VH3-23 and VK3-15, and comparison of the paratopes showed that the NWP and Y58 motifs of FISW84 similarly form an aromatic pocket but orient towards the fusion peptide slightly differently than 222-1C06 (Extended Data Fig. 4l). Molecular dynamics simulations also showed that VH3-23-utilizing and VH3-30-utilizing mAbs from our study and FISW84 targeted the HA fusion peptide similarly to the cryo-EM structure of 222-1C06 via the aromatic pocket created by the K-CDR3 NWP and H-CDR2 Y58 motifs, albeit at different orientations (Extended Data Fig. 4m). Crucially, molecular dynamic and cryo-EM analyses revealed numerous intra-Fab interactions of hydrophobic and aromatic amino acids, including p-stacking of K-CDR3-P95 with K-CDR3-W94 and H-CDR2-Y58 that rigidified the paratope (Extended Data Fig. 4n, o, Supplementary Table 3).
Broad analysis of human, swine and avian H1 viruses revealed that the side-chain contacts of 222-1C06 were highly conserved (94–100% conserved; Fig. 2j). In addition, the side-chain contacts were 100% conserved across 100 years of H1N1 virus evolution in humans (Extended Data Fig. 4p). Deep mutational scanning of potential H1 viruses at these contact residues indicated substantial permissibility (Extended Data Fig. 4q), although these mutations appear to not have been selected for in nature (Fig. 2j). The five side-chain contacts of this broad epitope were also highly conserved across group 1 viruses, with the W343 contact being 100% conserved across all group 1 viruses (Fig. 2k). Together, these data reveal that B cells targeting the anchor epitope utilized a highly restricted V(D)J gene repertoire, and the specific features within this repertoire made critical and extensive contacts with the conserved anchor epitope.
The anchor epitope is a common target
Owing to the restricted repertoire features of anchor-targeting mAbs, we next determined the relative proportion of B cells with these features by interrogating single-cell repertoire sequencing of HA-specific B cells isolated from 20 human participants following cHA vaccination4,5. The cHA vaccine platform is intended to specifically induce antibodies to the stalk domain by retaining the stalk domain of H1 and replacing the head domain of HA with that of an avian subtype, either H8 (prime) or H5 (boost) for this trial4,5. To investigate the proportion of B cells with anchor epitope-binding repertoire features, we selected B cells that used VH3-23/VH3-30/VH3-30-3/VH3-48, VK3-11/VK3-15, JK4/JK5, a K-CDR3 ten amino acids in length, and possessed an NWP motif within the K-CDR3. We also segregated B cells expressing VH1-69 and a κ-chain, which are commonly used by B cells that target the CS epitope. We identified that B cells with features of antibodies binding the anchor epitope were abundant within the human B cell repertoire, with 6% of all B cells identified fitting within this defined repertoire (Fig. 3a). Moreover, all but one participant had at least one B cell with anchor-binding repertoire features (Fig. 3b). In addition, 32 out of 33 mAbs generated from the selected anchor-targeting B cell list competed for HA binding with 047-09 4F04 (Extended Data Fig. 5a), indicating that this population was greatly enriched for anchor-targeting B cells. Moreover, the anchor-binding B cells were highly mutated and were largely class-switched to IgG1 (Extended Data Fig. 5b, c), suggesting that these B cells were MBCs that had undergone affinity maturation and class-switch recombination. Together, these data indicate that the anchor epitope is a common target of the human MBC repertoire following cHA vaccination.
a, Proportion of all cH5/1+ B cells with repertoire features of anchor-binding mAbs, VH1-69/κ (CS epitope) or with other repertoire features. The number in the centre of the pie graph is the number of B cells analysed. b, Proportion of B cells with anchor-binding mAb features or that use VH1-69/κ-chain by participant (n = 20 donors). Lines connect the same participant. Data were analysed using a two-tailed paired non-parametric Wilcoxon matched-pairs signed rank test. c, Electron microscopy polyclonal epitope mapping (EMPEM) summary of polyclonal antibodies (pAbs) binding to A/Michigan/45/2015 HA in the serum of participants 236 and 241 collected at day 7 and day 14 following 2014 quadrivalent inactivated influenza vaccine. Fabs shown as graphics with dotted lines represent predicted placements due to limited particle representation. 2D class averages were imaged at ×62,000 normal magnification d, Overlap of 241 IgA 2F04 Fab and pAb binding the anchor epitope from participant 241. See also Extended Data Fig. 5.
To confirm that anchor epitope-targeting mAbs were representative of the serum antibody response, we performed electron microscopy polyclonal epitope mapping (EMPEM)20 with serum antibodies from participants 236 and 241 from the 2014 quadrivalent inactivated influenza vaccine cohort (Extended Data Table 1). Both participants had detectable antibodies targeting the anchor epitope at days 7 and 14 post-vaccination (Fig. 3c, Extended Data Fig. 5d, e). Comparison of anchor epitope-binding polyclonal antibodies identified in participant 241 revealed that the 241 IgA 2F04 mAb strongly overlapped with the 241 polyclonal antibody (Fig. 3d), suggesting that the polyclonal antibody derived from this clonal expansion. Together, these data indicate that humoral immunity against the anchor epitope is common within the MBC pool and polyclonal serum antibody response after vaccination.
cHA induces anchor-binding antibodies
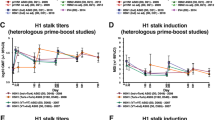
To investigate whether participants enrolled in a phase I clinical trial of the cHA vaccine (Fig. 4a) mounted an antibody response to the anchor and the CS epitope, we adapted the competition ELISA to detect serum antibody responses that could compete for binding with 047-09 4F04 and CR9114, respectively. Three different vaccine formulations were used in this trial, with participants being primed with either a cH8/1 inactivated influenza vaccine (IIV) with an oil-in-water adjuvant (AS03) or a cH8/1 live-attenuated influenza vaccine followed by a boost with a cH5/1 IIV with or without AS03 (Fig. 4a). Only participants who received the IIV + AS03 on either the prime or boost were capable of seroconverting against both the anchor and the CS epitopes (Fig. 4b, c, Extended Data Fig. 6a, b). Participants who received the cH8/1 IIV + AS03 prime did not further increase serum antibodies to either stalk epitope after the cH5/1 + AS03 boost (Fig. 4c), suggesting that these B cells were refractory to continued activation. Serum titres against the anchor and CS epitopes (Extended Data Fig. 6a, b) closely matched that of serum neutralizing titres against a cH6/1N8 virus and an avian-swine H1N1 virus5, suggesting that the anchor-targeting and CS-targeting serum antibodies were responsible for neutralization.
a, cHA vaccine trial design including vaccine groups (right; group 1 n = 10 participants; group 2 n = 7 participants; group 4 n = 7 participants; group 3 and 5 n = 6). i.m., intramuscular; i.n., intranasal; LAIV, live-attenuated influenza vaccine; PBS, phosphate buffered saline. Bottle images created with BioRender.com. b, c, Fold change by participant of serum antibodies competing for binding to the anchor and CS epitopes after the prime (d29/d1; b) and the boost (d113/85; c). d, e, Proportion of participants who seroconverted (d) and half-maximal effective concentration titres (EC50; e) to the anchor and CS epitopes. Individuals in the cHA (IIV + AS03) cohort were those who received the cHA vaccine with adjuvant (cH8/1 IIV + AS03 prime and cH5/1 IIV + AS03 boost; n = 17 donors) and the IIV cohort were those who received licensed IIV vaccines (2009 monovalent inactivated influenza vaccine, 2010 trivalent inactivated influenza vaccine and 2014 quadrivalent inactivated influenza vaccine; n = 11 donors). Data in b, c and e are mean ± s.d. The dotted line represents the limit of detection. Data in b and c were analysed by two-tailed non-parametric Kruskal–Wallis tests. Data in d were analysed by Fisher’s exact test. Data in e were analysed by two-tailed unpaired non-parametric Mann–Whitney test. See also Extended Data Fig. 6.
All but one participant in the IIV + AS03 groups seroconverted against the anchor epitope (Fig. 4d) and had higher titres against the anchor epitope than participants who received the 2009 monovalent inactivated influenza vaccine or seasonal influenza virus vaccines (Fig. 4e). However, the precise role of the cHA immunogen versus the AS03 adjuvant in inducing anti-stalk antibody responses could not be resolved in our study. Notably, those individuals who received the IIV alone had weak plasmablast responses relative to those individuals who received the IIV + AS03 (ref. 4), suggesting that the oil-in-water adjuvant, not the cHA immunogen, was essential for robust activation of B cells and anti-anchor antibody responses. Moreover, considerable pre-existing antibodies may hinder recall of B cells to the stalk. Individuals first exposed to the 2009 pH1N1, a virus for which individuals had low pre-existing humoral immunity, had proportionally more isolated mAbs to the stalk and were more likely to have an anti-anchor mAb than individuals who had repeatedly been exposed to the pH1N1 virus in subsequent influenza seasons (Extended Data Fig. 6c, d), therefore suggesting that pre-existing antibodies may limit antibody responses to the HA stalk. Despite robust recall of antibodies to the anchor and CS epitopes by the adjuvanted vaccines, titres decreased 1 year after vaccination (day 420; Extended Data Fig. 6e, f). Together, these data indicate that the adjuvanted inactivated cHA vaccine can robustly induce antibodies to multiple stalk epitopes, including the anchor.
Headless HA antigens, including mini-HA21, are attractive universal influenza virus vaccine antigens, as these antigens lack the immunodominant epitopes of the HA head21,22. However, only 1 out of 50 anchor-binding mAbs bound the mini-HA antigen21, whereas all anchor epitope-binding mAbs bound cH6/1 (Extended Data Fig. 6g). Compared to full-length HA, the membrane-proximal region of the mini-HA splays by approximately 14.5 Å (ref. 21), which may disrupt the antigenicity of the anchor epitope. To understand whether anchor epitope-targeting antibodies could bind to the mini-HA in a more native setting, we generated a membrane-bound mini-HA and observed that mAbs targeting the anchor and CS epitopes readily bind to both the full-length membrane-bound A/California/7/2009 HA and the membrane-bound mini-HA (Extended Data Fig. 6h), indicating that the mini-HA is antigenic when natively presented. Furthermore, we demonstrated that anchor epitope-targeting antibodies bound HA with a fibritin but not a GCN4 trimerization domain (Extended Data Fig. 6i), highlighting selection of the trimerization domain as an important factor for vaccine design. Together, these data demonstrate that native-like HA antigens, such as the cHA vaccine, can recall MBCs that target the anchor epitope.
Discussion
In this study, we identified a public class of bnAbs that target an epitope at the anchor of the HA stalk domain near the membrane. The anchor-targeting mAbs were public clonotypes across participants, with all antibodies possessing two conserved, germline-encoded binding motifs: a Y58 directly following the H-CDR2 and an NWP motif within the K-CDR3. The neutralizing activity of anchor epitope-targeting mAbs to pre-pH1N1 and post-pH1N1 viruses and a swine-origin H1-expressing virus indicates that the anchor epitope is an important target for pan-subtype neutralizing antibodies. As two of the last four influenza virus pandemics were caused by H1N1 viruses and a recent report has shown that antigenically novel H1-expressing viruses commonly spill over from swine into humans23, it is critically important to generate pan-H1 vaccines to prevent the next influenza pandemic. Moreover, the ability of anti-anchor antibodies to neutralize an H2 virus and the general conservation of contact residues suggests that anchor-targeting antibodies have the potential to acquire cross-neutralization against group 1 viruses.
Our study highlights an additional broadly protective epitope of the HA stalk and provides guidance on how vaccines can be designed to drive broadly protective antibodies to multiple distinct epitopes, which can work cooperatively to provide optimal protection while avoiding the generation of antibody escape mutants. Notably, studies in the HIV-1 field have shown that bnAb monotherapy can lead to the develop-ment of antibody-resistant viral variants24,25,26, whereas combination bnAb therapy does not27. In addition, immune focusing towards a single epitope can lead to the generation and selection of viral escape mutants at these highly conserved epitopes2,3,28,29. Therefore, it is critical that future universal influenza virus vaccines elicit antibodies to multiple conserved epitopes to prevent the generation of bnAb escape viruses.
The angle of approach of anchor-binding mAbs and the proximity of the epitope to the viral membrane suggest that this epitope is typically obstructed, limiting antibody recognition and B cell activation. However, membrane-bound HA typically flexes between 0° and 25° and up to 52° on its threefold axis11, suggesting antibodies and B cells can easily access the anchor epitope during this flexing process. Moreover, H1 viruses possess a highly conserved glycosylation site on the HA stalk that lies above the anchor epitope11,30, which may obstruct antibodies from targeting this epitope from above. Similarly, a neutralizing mAb to the Middle East respiratory syndrome coronavirus targets an epitope on S2 from an upward angle to avoid glycans31. Therefore, the upward angle of approach may be a common feature of antibodies that recognize epitopes below glycans.
Our study shows that humans have pre-existing immunity against the anchor epitope and influenza virus vaccination can recall MBCs to secrete antibodies to this epitope. However, vaccine HA antigens must have a native-like conformation near the transmembrane domain, as trimer splaying potentially due to the GCN4 trimerization domain ablates antibody binding to the anchor epitope. Moreover, our study reveals that the cHA vaccine strategy recalled MBCs to the anchor and CS epitopes, as these MBCs do not need to compete against MBCs that target the immunodominant variable HA head epitopes3,9,17,32. Similarly, the mini-HA/headless HA vaccine strategy has the potential to also recall MBCs to multiple epitopes of the HA stalk domain, if displayed natively22,33. The addition of an oil-in-water AS03 adjuvant to the cHA was critical for recalling MBCs to the anchor epitope. Oil-in-water adjuvants largely function to emulsify antigen, which may prevent sequestration of antigen by circulating antibodies, increase delivery of free antigen to lymph nodes, and help to stimulate innate immune receptors34,35. Notably, an oil-in-water adjuvanted inactivated H5N1 vaccine induced higher neutralizing antibody titres, antibodies to more HA epitopes, and higher avidity antibodies36. Therefore, the inclusion of oil-in-water adjuvants may have an important role in generating bnAbs and may improve vaccine effectiveness of seasonal influenza vaccines. Together, our study shows that influenza vaccination strategies, such as the cHA vaccine with the AS03 adjuvant, have the capability to robustly induce antibodies to the previously underappreciated anchor epitope and can provide broad protection against H1 viruses.
Methods
Study approvals, cohorts and human materials
Human peripheral blood mononuclear cells (PBMCs) and serum were obtained from multiple donors from multiple cohorts, which is outlined in Extended Data Table 1. Informed consent was obtained from all participants. All studies were performed with the approval of the University of Chicago Institutional Review Board (IRB; ID #09-043-A). The 2009 pH1N1 infection and 2009 monovalent inactivated influenza vaccine (MIV) cohorts were also approved by the IRB at Emory University. The chimeric HA vaccine study cohort is identified as clinical trial NCT03300050 and further details on trial design are outlined elsewhere4,5. The study was approved by IRBs at local clinical sites, including Icahn School of Medicine at Mount Sinai, Duke University, and Cincinnati Children’s Hospital Medical Center. All experiments performed with mice were done in accordance with the University of Chicago and Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committees.
Cell lines
Human embryonic kidney HEK293T (female, #CRL-11268), Madin Darby canine kidney (MDCK; female, #CCL-34, NBL-2) and human A549 (#CCL-185) cells were purchased and authenticated by the American Type Culture Collection (ATCC). MDCK-SIAT1 cells were generated previously37. All cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C. HEK293T cells were maintained in advanced-DMEM supplemented with 2% ultra-low IgG fetal bovine serum (FBS; Invitrogen), 1% l-glutamine (Invitrogen) and 1% antibiotic-antimycotic (Invitrogen). MDCK, MDCK-SIAT1 and A549 cells were maintained in DMEM supplemented with 10% FBS (Invitrogen), 1% l-glutamine (Invitrogen) and 1% penicillin–streptomycin (Invitrogen). Jurkat cells expressing FcgRIIIa and FcgRI with a NFAT-driven luciferase reporter gene (#G7010) were acquired and validated by Promega and were directly used for antibody-dependent cellular cytotoxicity (ADCC) assays. Cell lines were not authenticated after receiving from suppliers and were not tested for mycoplasma.
Monoclonal antibody production
Monoclonal antibodies were generated as previously described38,39,40. Peripheral blood was obtained from each donor approximately 7 days after vaccination or infection or obtained 28+ days post-vaccination. Lymphocytes were isolated and enriched for B cells using RosetteSep. Total plasmablasts (CD3−CD19+CD27hiCD38hi; all cohorts except 2014 quadrivalent inactivated influenza vaccine (QIV)), IgG+ plasmablasts (CD3−CD19+IgM−CD27hiCD38hiIgG+IgA−; 2014 QIV), IgA+ plasmablasts (CD3−CD19+IgM−CD27hiCD38hiIgG−IgA+; 2014 QIV cohort), or HA+ bait-sorted MBCs (CD3−CD19+CD27+CD38lo/+HA+; for 030-09M 1B06) were single-cell sorted into 96-well plates. Genes encoding immunoglobulin heavy and light chains were amplified by reverse transcriptase PCR (RT–PCR), sequenced, cloned into human IgG1, human κ-chain, or human λ expression vectors, and co-transfected into HEK293T cells. Secreted mAbs were purified from the supernatant using protein A agarose beads. For mAbs generated from the 2014 QIV cohort, mAb names include the original isotype of the sorted plasmablast, and all mAbs were expressed as human IgG1. cH5/1-binding B cells (CD19+CD27+cH5/1+) were sorted from donors 28 days after cH5/1 vaccination (NCT03300050). Cells were sorted with A/California/04/2009 HA probe (for 030-09M 1B06) or cH5/1 probe, each with a Y98F mutation to ablate non-specific binding to sialic acids on B cells. mAb heavy chain and light chain sequences were synthesized from single-cell RNA sequencing data of cH5/1-baited B cells (IDT), and cloned into the human IgG1, human κ-chain or human λ expression vectors. B cell clones were determined by aligning all the V(D)J sequences sharing identical progenitor sequences, as predicted by IgBLAST using our in-house software, VGenes. Consensus sequence analysis was performed using WebLogo41 and sequence alignments were determined using Clustal Omega.
Viruses and recombinant proteins
Influenza viruses used in all assays were grown in-house in specific pathogen free (SPF) eggs, harvested, purified and titred. Recombinant HA, cHA and mini-HA were obtained from BEI Resources or generated in-house. Recombinant HA mutant proteins used in Extended Data Fig. 2 were generated with identified mutations from the deep mutational scanning experiments (see below) or with known mutations that have arisen naturally or were identified in other studies2,10,42,43,44,45,46,47,48,49,50,51,52 (Extended Data Table 3). All mutations were made on HA from A/California/7/2009 and were expressed in HEK293T cells and purified using Ni-NTA agarose beads (Qiagen).
Antigen-specific ELISA
High protein-binding microtitre plates (Costar) were coated with 8 haemagglutination units (HAU) of virus in carbonate buffer or with recombinant HA, including HA mutants described below, at 2 μg ml−1 in phosphate-buffered saline (PBS) overnight at 4 °C. Plates were washed the next morning with PBS 0.05% Tween and blocked with PBS containing 20% FBS for 1 h at 37 °C. Antibodies were then serially diluted 1:3 starting at 10 μg ml−1 and incubated for 1.5 h at 37 °C. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG antibody diluted 1:1,000 (Jackson Immuno Research) was used to detect binding of mAbs, and plates were subsequently developed with Super Aquablue ELISA substrate (eBiosciences). Absorbance was measured at 405 nm on a microplate spectrophotometer (Bio-Rad). To standardize the assays, control antibodies with known binding characteristics were included on each plate, and the plates were developed when the absorbance of the control reached 3.0 optical density (OD) units. All ELISAs were performed in duplicate twice. To define antibodies as targeting the H1 stalk, mAbs were tested for binding to cH5/1, which utilizes the head domain from H5-expressing viruses and the stalk domain from the pH1N1 virus53, and for haemagglutination inhibition (HAI) activity against pH1N1 (A/California/7/2009). mAbs that bound the cHA and lacked HAI activity were classified as those binding the HA stalk domain. To classify antigen specificity, mAbs that did not definitively bind to the HA head or stalk are listed as binding unknown HA+ epitopes. Affinity measurements, as represented as dissociation constant (Kd) at a molar concentration (M), were calculated using Prism 9 (GraphPad) by performing a non-linear regression. All experiments were performed in duplicate and technically replicated twice.
Competition ELISAs
Plates were coated with 50 µl of A/California/7/2009 HA at a concentration of 1 µg ml−1 and incubated overnight at 4 °C. To biotinylate the antibodies with known epitope specificities, CR9114 (CS epitope) and 047-09-4F04 (anchor epitope) were incubated at 4 °C with EZ-Link Sulfo-NHS-Biotin (Thermo Scientific) for 24 h or 48 h before use, respectively. After blocking the plates with PBS containing 20% FBS for 1 h at 37 °C, serum samples were incubated (starting dilution of 1:50 for human serum or 20 μg ml−1 for mAbs) in the coated wells for 2 h at room temperature. Either biotinylated CR9114 or 047-09 4F04 was then added at a concentration equal to twice its Kd and incubated in the wells with the serum or mAbs for 2 h at room temperature. The biotin-ylated antibodies were desalted before addition to remove free biotin using Zeba spin desalting columns, 7 k MWCO (Thermo Scientific). After washing the plates, wells were incubated with HRP-conjugated streptavidin (Southern Biotech) at 37 °C for 1 h for detection of the biotin-ylated antibody. Super Aquablue ELISA substrate (eBiosciences) was then added, and absorbance was measured at 405 nm on a microplate spectrophotometer (Bio-Rad). To standardize the assays, biotinylated CR9114 or 047-09 4F04 was incubated in designated wells on each plate without any competing serum or mAb, and data were recorded when the absorbance of these wells reached an OD of 1–1.5 units. After subtracting background, percent competition by serum samples was then determined by dividing the observed OD of a sample by the OD reached by the positive control, subtracting this value from 1, and multiplying by 100. For the serum data, ODs were log transformed and analysed by non-linear regression to determine EC50 values using Prism software (GraphPad). For Fig. 4 and Extended Data Fig. 5, only donors with serum for all timepoints were included. All experiments were performed in duplicate and technically replicated twice.
Polyreactive ELISAs
High-protein binding microtitre plates (Costar) were coated with 10 μg ml−1 calf thymus double-stranded DNA (dsDNA; Thermo Fisher Scientific), 2 μg ml−1 Salmonella enterica serovar Typhimurium flagellin (Invitrogen), 5 μg ml−1 human insulin (Sigma-Aldrich), 10 μg ml−1 KLH (Invitrogen) and 10 μg ml−1 Escherichia coli LPS (Sigma-Aldrich) in PBS. Plates were coated with 10 μg ml−1 cardiolipin in 100% ethanol and allowed to dry overnight. Plates were washed with water and blocked with PBS, 0.05% Tween and 1 mM EDTA. mAbs were diluted 1 μg ml−1 in PBS and serially diluted fourfold and added to plates for 1.5 h. Plates were washed and goat anti-human IgG-HRP (Jackson Immunoresearch) was diluted 1:2,000 in PBS, 0.05% Tween and 1 mM EDTA. Plates were washed with water and were blocked with PBS, 0.05% Tween and 1 mM EDTA for 5 min. Plates were washed again with water and were developed with Super Aquablue ELISA substrate (eBioscience) until the positive control mAb, 3H9 (ref. 54), reached an A450 of 3. All experiments were performed in duplicate and technically replicated twice.
Deep mutational scanning for stalk domain mutants
The mutant libraries used herein were previously described55. The libraries consist of all single amino acid mutations to A/WSN/1933 (H1N1). The experiments were performed by using biological triplicate libraries. The mutational antigenic profiling of 045-09 2B06, a CS epitope-binding mAb, was performed as previously outlined56. In brief, 106 tissue culture infectious dose 50 (TCID50) of two of the virus library biological replicates was diluted in 1 ml in IGM (Opti-MEM supplemented with 0.01% FBS, 0.3% BSA and 100 mg ml−1 calcium chloride) and incubated with an equal volume of 045-09 2B06 antibody at a final concentration of 50 or 25 μg ml−1 for 1.5 h at 37 °C. MDCK-SIAT1 cells were infected with the virus antibody mixtures. Two hours post-infection, the media were removed, the cells washed with 1 ml PBS, and 2 ml of fresh IGM was added. Fifteen hours post-infection, viral RNA was extracted, reverse-transcribed using primers WSNHA-For (5′-AGCAAAAGCAGGGGAAAATAAAAACAAC-3′) and WSNHA-Rev (5′-AGTAGAAACAAGGGTGTTTTTCCTTATATTTCTG-3′), and PCR amplified according to the barcoded-subamplicon library preparation as previously described55. The overall fraction of virions that survive antibody neutralization was estimated using quantitative RT–PCR (qRT–PCR) targeting the viral nucleoprotein (NP) and cellular GAPDH as previously described56. Using tenfold serial dilutions of the virus libraries, we infected cells with no antibody selection to serve as a standard curve of infectivity. qPCR Ct values from the standard curve samples compared to the virus–antibody mix samples were determined for NP and GAPDH. We then generated a linear regression to fit the difference between the NP and GAPDH Ct values for the standard curve samples, and then used this curve to interpolate the fraction surviving for the antibody–virus selection samples. Across the three library replicates, the fraction of virus surviving antibody selection was 0.17, 0.1 and 0.14.
Illumina(R) deep sequencing data were analysed using dms_tools2 version 2.4.12 software package57, which can be found at https://github.com/jbloomlab/dms_tools2. The computer code used is at https://github.com/jbloomlab/2B06_DMS, and the Jupyter notebook that performed most of the analysis is at https://github.com/jbloomlab/2B06_DMS/blob/master/analysis_notebook.ipynb. The sequencing counts were processed to estimate the differential selection for each mutation, which is the log enrichment of that mutation in the antibody-selected condition versus the control56. The numerical measurements of the differential selection that 2B06 imposes on each mutation can be found at: https://github.com/jbloomlab/2B06_DMS/blob/master/results/diffsel/tidy_diffsel.csv.
Deep mutational scanning for H1 variants
Amino acid preferences for the HA of A/WSN/1933 (H1N1) were previously determined55. In brief, deep mutational scanning was performed by passaging virus libraries at a low multiplicity of infection in MDCK-SIAT1 cells. Following deep sequencing of the resulting virus, amino acid preferences were determined using the Python package dms_tools (http://jbloomlab.github.io/dms_tools/), version 1.1.12. This program aligns subamplicon reads to the reference HA sequence, counts the number of mutations at each amino acid site, and determines amino acid preferences based on the mutation counts pre-selection and post-selection.
Microneutralization assays
Microneutralization assays for mAb characterization were carried out as previously described58,59. MDCK cells were maintained in DMEM supplemented with 10% FBS, 1% penicillin–streptomycin, and 1% l-glutamine at 37 °C with 5% CO2. The day before the experiment, 25,000 MDCK cells were added to each well of a 96-well plate. Serial twofold dilutions of mAb were mixed with an equal volume of 100 TCID50 of virus for 1 h and added to MDCK cells for 1 h at 37 °C. The mixture was removed, and cells were cultured for 20 h at 37 °C with 1X MEM supplemented with 1 μg ml−1 tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin and appropriate mAb concentration. Cells were washed twice with PBS, fixed with 80% ice-cold acetone at −20 °C for at least 1 h, washed three times with PBS, blocked for 30 min with 3% BSA–PBS, and then treated for 30 min with 2% H2O2. Cells were incubated with a mouse anti-NP antibody (1:1,000; Millipore) in 3% BSA–PBS for 1 h at room temperature, followed by goat anti-mouse IgG HRP (1:1,000; Southern Biotech) in 3% BSA–PBS for 1 h at room temperature. The plates were developed with Super Aquablue ELISA substrate at 405 nm until virus-only controls reached an OD of 1. The signal from uninfected wells was averaged to represent 100% inhibition. The signal from infected wells without mAb was averaged to represent 0% inhibition. Duplication wells were used to calculate the mean and s.d. of neutralization, and the half-maximal inhibitory concentration (IC50) was determined by a sigmoidal dose–response curve. The inhibition ratio (%) was calculated as: ((OD positive control – OD sample)/(OD positive control – OD negative control)) × 100. The final IC50 was determined using Prism software (GraphPad). All experiments were performed in duplicate and technically replicated twice.
H2N2 neutralization assays
Twenty thousand MDCK cells were seeded per well in a 96-well cell culture plate (Corning) and the cells were used the next morning to perform the neutralization assay. Antibody dilutions were prepared starting at 30 μg ml−1 with threefold subsequent dilutions in 1X MEM. Each respective dilution was mixed with 10,000 plaque-forming units (PFU) of cold-adapted A/Ann Arbor/6/1960 (H2N2) virus for 1 h at room temperature. After 1 h, cells were washed with PBS and 100 μl of antibody–virus mixture was added onto the cells for 1 h at 37 °C. Next, the antibody–virus mixture was removed and 60 μl of 1X MEM containing TPCK was added to each well. Of each corresponding antibody dilution, 60 μl was also added to each well and the cells were incubated at 33 °C for 3 days. On the third day, a haemagglutination assay was performed using turkey red blood cells to assess the HAU at each antibody dilution.
In vivo challenge infections
mAb cocktails (Extended Data Fig. 2b) were passively transferred into 6–8-week-old female BALB/c mice (Jackson Laboratories) by intraperitoneal injection of 0.2, 1 and 5 mg per kg mAb cocktail, which are further detailed in Supplementary Table 1. Negative control mice received 5 mg per kg of the anthrax-specific mAb 003-15D03 as an isotype control. mAbs were administered 2 h before infection for prophylactic treatment and 48 h post-infection for therapeutic treatment. For prophylactic mAb studies with A/Netherlands/602/2009 (Extended Data Fig. 3a), mice were anaesthetized with isoflurane and intranasally challenged with 10 lethal dose 50 (LD50) of mouse-adapted A/Netherlands/602/2009 H1N1 virus, with 10 μl of virus administered into each nostril (20 μl total). For therapeutic treatment of A/Netherlands/602/2009 and prophylactic treatment of A/Fort Monmouth/1/1947, mice were anaesthetized with a ketamine–xylazine–water cocktail (0.15 mg ketamine per kg and 0.03 mg per kg xylazine; 100 ml intraperitoneally) and infected with 10 LD50 of A/Netherlands/602/2009 or A/Fort Monmouth/1/1947. As a read out, survival and weight loss were monitored 1–2 times daily for 2 weeks. Mice wereeuthanized upon 25% weight loss or at the end of the experiment (14 days post-challenge). Five mice per condition per experiment with two biological replicates were utilized based on a previously performed power analysis. Data were pooled for analysis.
To determine differences in lung viral load, 5 mg per kg of the antibody cocktails was administered prophylactically as described above. Two hours after mAb administration, mice (n = 5 mice per group) were anaesthetized and intranasally challenged with 1 LD50 of A/Netherlands/602/2009. Lungs were collected at day 3 and day 6 post-infection, homogenized and viral load was determined via plaque assay. All experiments were done in accordance with the University of Chicago and Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committees. Animals studies were not blinded or randomized.
Plaque assay
For determination of viral load in mouse lung tissues a standard plaque assay was performed. Confluent monolayers of MDCK cells were infected with serial dilutions of homogenized lung tissue ranging from 1:10 to 1:1,000,000 diluted in 1X MEM (1% penicillin–streptomycin antibiotics mix, 1% HEPES, 1% l-glutamine and 1% sodium-bicarbonate (Gibco)) for 1 h at 33 °C, with shaking every 15 min. Afterwards, an overlay containing 2% Oxoid agar (Thermo Fisher), H2O, 2X MEM, DEAE and TPCK-treated trypsin was added to the cells. The plates were incubated at 33 °C for 3 days and then fixed with 3.7% paraformaldehyde overnight at 4 °C. Plaques were visualized by immunostaining. Here, the agar overlay was removed and the plates blocked with 3% milk and PBS. The blocking solution was removed and primary antibody ((H1N1 guinea pig anti-sera (generated in house)) diluted 1:3,000 in 1% milk and PBS was added for 1 h. The plates were washed three times with PBS and secondary antibody (anti-mouse IgG H&L peroxidase-conjugated (Rockland) diluted 1:3,000 in 1% milk and PBS was added for 1 h. The plates were washed three times with PBS and developed by using KPL TrueBlue Peroxidase Substrate (SeraCare).
Antibody-dependent cellular cytotoxicity reporter assay
A549 cells were maintained in DMEM supplemented with 10% FBS, 10 U ml−1 penicillin, and 10 mg ml−1 streptomycin) and were plated in 96-well, white-walled plates (Costar) at 2.5 × 105 cells per ml overnight at 37 °C with 5% CO2. The following day, cells were washed with PBS and infected with A/Netherlands/602/2009 at a multiplicity of infection of 5 in UltraMDCK media (Lonza) for 24 h in the absence of TPCK-treated trypsin. mAbs were serially diluted in assay buffer (RPMI 1640 supplemented with 4% ultra-low IgG FBS; Gibco), starting at 60 μg ml−1 and diluted threefold. Cell medium was aspirated and 25 μl of assay buffer and 25 μl of diluted antibody were added to each well. Jurkat cells expressing human FcgRIIIa with a NFAT-driven luciferase reporter gene (Promega) were diluted to 3 × 106 cells per ml, 25 μl of cells was added to each well and incubated at 37 °C with 5% CO2 for 6 h. Plates were removed from the incubator and placed at room temperature for 15 min. Of the BioGlo luciferase substrate (Promega), 75 μl was added to each well and luminescence was read immediately using a Syngery H1 hybrid multimode microplate reader (Biotek). EC50 values were determined using Prism 8 (GraphPad).
HA–antibody binding footprint mapping
The footprints of three mAbs (FISW84 (PDB: 6HJQ), CR9114 (PDB: 4FQI) and FI6v3 (PDB: 3ZTN)) were mapped onto one HA protomer (A/California/4/2009, PDB: 4M4Y) using UCSF Chimera60 and Adobe Photoshop. Negative-stain EM maps of HA–Fab complexes were aligned in UCSF Chimera and estimated footprints were mapped onto one HA protomer. Individual protomers of the HA trimer are indicated in different shades of grey.
Negative-stain EM
Immune complexes were prepared by incubating Fab with HA (A/California/04/2009 with E376K or E376G stabilizing mutations) at greater than 3:1 molar ratio for 2 h at room temperature. Samples were deposited at approximately 10 μg ml−1 on glow-discharged, carbon-coated 400 mesh copper grids (Electron Microscopy Sciences) and stained with 2% w/v uranyl formate. Samples were imaged at ×52,000 magnification, 120 kV, on a Tecnai Spirit T12 microscope equipped with an Eagle CCD 4k camera (FEI) or ×62,000 magnification, 200 kV, on a Tecnai T20 microscope equipped with a CMOS 4k camera (TVIPS). Micrographs were collected with Leginon, single particles were processed with Appion, Relion and XQuartz, and footprints were mapped with UCSF Chimera, and figures were made with UCSF Chimera60,61,62,63.
Cryo-EM
222-1C06 and 045-09 2B05 Fabs were incubated at greater than 3:1 molar ratio with HA (A/California/7/2009, E376K) for 1 h at room temperature. 045-09 2B05 Fab, targeting the lateral patch, was added to the immune complex to induce particle tumbling and increase angular sampling3. Using a Thermo Fisher Vitrobot, the immune complex (0.5 mg ml−1) incubated with lauryl maltose neopentyl glycol (5 µM, Anatrace) was deposited onto glow-discharged Au 1.2/1.3 300 mesh grids (Electron Microscopy Sciences), blotted for 7 s, and plunge-frozen in liquid ethane. Samples were imaged at ×36,000 nominal magnification on a 200 kV Talos Arctica electron microscope (FEI) with a CETA 4k CMOS camera (FEI, total dose 49.92 e/Å2) and Gatan K2 Summit detector in counting mode. 2,243 micrographs were collected, aligned and CTF-corrected using Leginon, MotionCor2 in Appion, and Patch-CTF in CryoSPARC2, respectively61,62,64,65. In CryoSPARC2, particles were picked using apo HA templates, selected through reference-free 2D classification, and reconstructed through 3D classification and refinement. The final map resolved to a global 3.75 Å resolution with C3 symmetry and 44,224 particles. Figures were made in Prism 8 (GraphPad) and UCSF Chimera60.
Model building and refinement
A predicted model of 222-1C06 Fab was generated using abYsis (http://www.abysis.org/abysis/) and docked into EM density along with an initial model of CA09 H1 HA + 045-09 2B05 (PDB: 7MEM). The initial model was iteratively refined using COOT and Rosetta66,67. The final model was numbered using the H3 and Kabat numbering schemes. The final model and map were evaluated using MolProbity, EMRinger68,69, Phenix and the PDB validation server. After modelling the immune complex, we segmented the Fab density from HA in the cryo-EM map and mapped the footprint of the 222-1C06 model in the HA density. Cryo-EM data collection and refinement statistics are included in Extended Data Table 4.
Electron microscopy polyclonal epitope mapping
Human serum samples were heat-inactivated at 55 °C for 30 min before incubating on Capture Select IgG-Fc (ms) Affinity Matrix (Fisher) to bind IgG at 4 °C for 72 h on a rotator. Samples with IgG bound to resin were centrifuged at 4,000 rpm and supernatant was collected. IgG samples were washed three times with PBS followed by centrifugation to remove supernatant. Samples were buffer exchanged into buffer containing 100 mM Tris, 2 mM EDTA and 10 mM l-cysteine through centrifugation with Amicon filters, then incubated with papain for 4 h at 37 °C, shaking at 80 rpm. The digestion reactions were quenched with 50 mM iodoacetamide, buffer exchanged to TBS and separated by size-exclusion chromatography (SEC) with a Superdex 200 increase 10/300 column (GE Healthcare). Fab and undigested IgG were collected and concentrated, and 500 μg Fab was complexed with 10 μg HA for 18 h at room temperature. Reactions were purified by SEC and immune complexes were collected and concentrated. Negative-stain EM grids were prepared as described above.
Membrane-bound HA and mAb staining
HEK293T cells were plated into a six-well plate and transfected overnight with 0.2 μg of plasmid and 10 μg ml−1 PEI. After 12–16 h, media were replaced with PFHM-II (Gibco) and cells were rested for 3 days. Transfected cells were trypsinized, washed and aliquoted. Cells were stained with 10 μg ml−1 of individual mAbs for 30 min. Cells were washed and stained with anti-human IgG Fc-BV421 for 30 min. Cells were washed two times and run on a BD LSRFortessa and collected with BD FACSDiva software. Data were analysed using FlowJo v10.
Single-cell RNA sequencing and repertoire analysis
cH5/1+ memory B cells (CD19+CD27+HA+) were bulk sorted and partitioned into nanolitre-scale gel bead-in-emulsions (GEMs) to achieve single-cell resolution using the 10x Genomics Chromium Controller and according to the manufacturer’s instruction (10x Genomics). The sorted single cells were processed according to 5′ gene expression and B cell immunoglobulin enrichment instruction to prepare the libraries for sequencing. Libraries were sequenced using an Illumina HiSeq 4000 at Northwestern University or an Illumina NextSeq 500 at the University of Chicago. Cellranger Single-Cell Software Suite (version 3.0) was used to perform sample demultiplexing, barcode processing, and single-cell 5′ and V(D)J counting, and Cellranger mkfastq was used to demultiplex raw base call (BCL) files into sample-specific fastq files. Subsequently, GRCh38-1.2.0 and cellranger-vdj-GRCh38-alta-ensembl-2.0.0 were used as references for the transcriptome and V(D)J assembly, respectively. Cellranger counts and Cellranger vdj package were used to identify gene expression and assemble V(D)J pairs of antibodies.
Single-cell datasets were analysed using Seurat 3 toolkit (Version 3.2.0). We performed conventional pre-process steps for all 20 donors including cell quality control (QC), normalization, identification of highly variable features, data scaling and linear dimensional reduction. More specifically, we only kept cells with more than 200 and less then 2,500 detected genes for the QC step. We also filtered out cells with high mitochondrial gene expression using a ‘softThreshold’ function in the R package LinQ-View (version 0.99)70. We normalized the RNA data using conventional log normalization. We identified 2,000 highly variable genes for each dataset and performed principle component analysis (PCA) in linear dimensional reduction step. We then integrated all 20 single-cell datasets from vaccinated participants to remove batch effects using the Anchor method in Seurat 3. In this analysis, we filtered our dataset and only kept cells with both transcriptome and full length and paired heavy and light chain V(D)J sequences (n = 1,952). From these cells, we identified a group of ‘VH1-69/κ’ B cells that used the VH1-69 gene and κ-light chain, which is enriched for B cells targeting the BN stalk epitope. We also identified a group of ‘anchor epitope’-specific B cells by the following rules: (1) VH locus: VH3-23, VH3-30, VH3-30-3 or VH-3-48; (2) VK locus: VK3-11 or VK3-15; (3) JK locus: JK4 or JK5; (4) K-CDR3 length equal to 10; and (5) a ‘NWP’ pattern in the K-CDR3 peptide.
HA conservation modelling
Pan-H1 conservation models are based on consensus strains (listed in Supplementary Table 4) of distinct H1 clades isolated from humans, swine and avian sources, as described in Zhuang et al.71 and inclusion of the Eurasian swine-like A/swine/Jiangsu/J004/2018 (ref. 23). To generate the group 1 HA conservation model, we selected one representative sequence for each group 1 HA subtype from FluDB (https://www.fludb.org/; Supplementary Table 5) according to a previous study72. A multiple sequence alignment from these HA protein sequences was generated using MUSCLE73 and the conservation of each residue was quantified using an entropy model41. Seasonal H1 conservation models are based on consensus strains of H1N1 viruses (59 strains total) circulating between 1918–1957 and 1976–2019, which was previously described3. Amino acid alignments and H3 numbering were performed using Librator74 and Burke and Smith HA numbering72.
Structure prediction
To predict the structures of the investigated Fv fragments (222-1C06, FISW84, 241 IgA 2F04 and SFV009 3G01) with A/California/4/2009 E47G HA (PDB: 7MEM), we applied the program RosettaAntibody67,75,76. The Fvs were protonated using the Protonate 3D tool77,78. Charge neutrality was ensured by utilizing the uniform background plasma approach in AMBER79,80. Using the tleap tool of the AmberTools20 (ref. 81) package, the structure models were soaked in cubic water boxes of TIP3P water molecules with a minimum wall distance of 10 Å to the protein82. Parameters for all antibody models derive from the AMBER force field 14SB83. The Fvs were carefully equilibrated using a multistep equilibration protocol84.
Metadynamics simulations
To enhance the sampling of the conformational space, well-tempered bias-exchange metadynamics85,86,87 simulations were performed in GROMACS88,89 with the PLUMED 2 implementation90. We chose metadynamics as it enhances sampling on predefined collective variables. The sampling is accelerated by a history-dependent bias potential, which is constructed in the space of the collective variables85,86,91. As collective variables, we used a well-established protocol, boosting a linear combination of sine and cosine of the ψ torsion angles of all six CDR loops calculated with functions MATHEVAL and COMBINE implemented in PLUMED 2 (ref. 90). As discussed previously, the ψ torsion angle captures conformational transitions comprehensively92. The underlying method presented in this paper has been validated in various studies against a large number of experimental results93. The simulations were performed at 300 K in an NpT ensemble using the GPU implementation of the pmemd module94 to be as close to the experimental conditions as possible and to obtain the correct density distributions of both protein and water. We used a Gaussian height of 10.0 kJ mol−1 and a width of 0.3 rad. Gaussian deposition occurred every 1,000 steps and a biasfactor of 10 was used. 500 ns of bias-exchange metadynamics simulations were performed for the prepared Fv structures. The resulting trajectories were aligned to the whole Fv and clustered with the program cpptraj80,95 using the average linkage hierarchical clustering algorithm with a RMSD cut-off criterion of 1.2 Å resulting in a large number of clusters. The cluster representatives for the antibody fragments were equilibrated and simulated for 100 ns using the AMBER 20 (ref. 81) simulation package.
Molecular dynamics simulations
Molecular dynamics simulations were performed in an NpT ensemble using the pmemd.cuda module of AMBER 20 (ref. 80). Bonds involving hydrogen atoms were restrained with the SHAKE algorithm96, allowing a time step of 2.0 fs. Atmospheric pressure (1 bar) of the system was set by weak coupling to an external bath using the Berendsen algorithm97. The Langevin thermostat98 was used to maintain the temperature during simulations at 300 K.
With the obtained trajectories, we performed a time-lagged independent component analysis (tICA) using the Python library PyEMMA 2, using a lag time of 10 ns. tICA was applied to identify the slowest movements of the investigated Fab fragments and consequently to obtain a kinetic discretization of the sampled conformational space99. On the basis of the tICA conformational spaces, thermodynamics and kinetics were calculated with a Markov-state model100 of all six CDR loops by using PyEMMA 2. The resulting kinetically dominant ensemble in solution was further used to predict the interactions of H1 with the Fvs. To model the complex and to predict interactions in the binding interface, we used the crystal structure of the full-length influenza HA (PDB: 7MEM) as template structure. In addition, the obtained complex structure was further minimized and equilibrated.
Statistical analysis
All statistical analyses were performed using Prism software (GraphPad versions 8 and 9) or R. Sample sizes (n) for the number of mAbs tested are indicated in corresponding figures or in the centre of pie graphs. The number of biological repeats for experiments and specific tests for statistical significance used are indicated in the corresponding figure legends. P values less than or equal to 0.05 were considered significant: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Repertoire data generated from single-cell RNA sequencing data were deposited at Mendeley Data (https://data.mendeley.com/datasets/jzsx489pmk/1). Accession numbers for all other anchor-targeting mAbs are included in Supplementary Table 6. Electron microscopy maps were deposited to the Electron Microscopy Data Bank under accession IDs: EMD-25634–EMD-25646. The cryo-EM map and model of anchor and lateral patch Fabs binding H1 HA were deposited to the RCSB database with accession numbers EMD-25655/PDB 7T3D. All next-generation sequencing data for 045-09 2B06 deep mutational scanning and for the H1N1 mutational scanning can be found on the Sequence Read Archive under BioProject accession number PRJNA309339. The following Protein Data Bank accession numbers were downloaded and included in the paper: 6HJQ, 3SDY, 4NM8, 4M4Y, 4WE4, 4JTV, 4FQI, 3ZTN and 7MEM. Sera from the vaccine cohorts are unique to this study and are not publicly available. All source data are included with the paper. All other material is available on reasonable request to the corresponding authors. Source data are provided with this paper.
References
Paules, C. I., Marston, H. D., Eisinger, R. W., Baltimore, D. & Fauci, A. S. The pathway to a universal influenza vaccine. Immunity 47, 599–603 (2017).
Park, J. K. et al. Pre-existing immunity to influenza virus hemagglutinin stalk might drive selection for antibody-escape mutant viruses in a human challenge model. Nat. Med. 26, 1240–1246 (2020).
Guthmiller, J. J. et al. First exposure to the pandemic H1N1 virus induced broadly neutralizing antibodies targeting hemagglutinin head epitopes. Sci. Transl. Med. 13, eabg453 (2021).
Bernstein, D. I. et al. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 20, 80–91 (2020).
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2020).
Ng, S. et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat. Med. 25, 962–967 (2019).
Aydillo, T. et al. Pre-existing hemagglutinin stalk antibodies correlate with protection of lower respiratory symptoms in flu-infected transplant patients. Cell Rep. Med. 1, 100130 (2020).
Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B cellresponse against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 (2011).
Andrews, S. F. et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 7, 316ra192 (2015).
Dreyfus, C. et al. Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012).
Benton, D. J. et al. Influenza hemagglutinin membrane anchor. Proc. Natl Acad. Sci. USA 115, 10112–10117 (2018).
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011).
Friesen, R. H. et al. A common solution to group 2 influenza virus neutralization. Proc. Natl Acad. Sci. USA 111, 445–450 (2014).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011).
DiLillo, D. J., Palese, P., Wilson, P. C. & Ravetch, J. V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 126, 605–610 (2016).
He, W. et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl Acad. Sci. USA 113, 11931–11936 (2016).
Guthmiller, J. J. et al. Polyreactive broadly neutralizing B cells are selected to provide defense against pandemic threat influenza viruses. Immunity 53, 1230–1244.e5 (2020).
Sutton, T. C. et al. In vitro neutralization is not predictive of prophylactic efficacy of broadly neutralizing monoclonal antibodies CR6261 and CR9114 against lethal H2 influenza virus challenge in mice. J. Virol. 91, e01603-17 (2017).
Benton, D. J., Gamblin, S. J., Rosenthal, P. B. & Skehel, J. J. Structural transitions in influenza haemagglutinin at membrane fusion pH. Nature 583, 150–153 (2020).
Han, J. et al. Polyclonal epitope mapping reveals temporal dynamics and diversity of human antibody responses to H5N1 vaccination. Cell Rep. 34, 108682 (2021).
Impagliazzo, A. et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015).
Yassine, H. M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Sun, H. et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl Acad. Sci. USA 117, 17204–17210(2020).
Caskey, M. et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015).
Caskey, M. et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 23, 185–191 (2017).
Bar, K. J. et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N. Engl. J. Med. 375, 2037–2050 (2016).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484 (2018).
Raymond, D. D. et al. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl Acad. Sci. USA 115, 168–173 (2018).
Linderman, S. L. et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc. Natl Acad. Sci. USA 111, 15798–15803 (2014).
Tate, M. D. et al. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6, 1294–1316 (2014).
Pallesen, J. et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl Acad. Sci. USA 114, E7348–E7357 (2017).
Dugan, H. L. et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci. Transl. Med. 12, eabd3601 (2020).
van der Lubbe, J. E. M. et al. Mini-hemagglutinin vaccination induces cross-reactive antibodies in pre-exposed NHP that protect mice against lethal influenza challenge. NPJ Vaccines 3, 25 (2018).
Cantisani, R. et al. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J. Immunol. 194, 1717–1725 (2015).
Liang, F. et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 9, eaal2094 (2017).
Khurana, S. et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2, 15ra15 (2010).
Bloom, J. D., Gong, L. I. & Baltimore, D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328, 1272–1275 (2010).
Guthmiller, J. J., Dugan, H. L., Neu, K. E., Lan, L. Y. & Wilson, P. C. An efficient method to generate monoclonal antibodies from human B cells. Methods Mol. Biol. 1904, 109–145 (2019).
Wrammert, J. et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453, 667–671 (2008).
Smith, K. et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 4, 372–384 (2009).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Doud, M. B., Lee, J. M. & Bloom, J. D. How single mutations affect viral escape from broad and narrow antibodies to H1 influenza hemagglutinin. Nat. Commun. 9, 1386 (2018).
Lang, S. et al. Antibody 27F3 broadly targets influenza A group 1 and 2 hemagglutinins through a further variation in VH1-69 antibody orientation on the HA stem. Cell Rep. 20, 2935–2943 (2017).
Xu, R. et al. Functional balance of the hemagglutinin and neuraminidase activities accompanies the emergence of the 2009 H1N1 influenza pandemic. J. Virol. 86, 9221–9232 (2012).
Fu, Y. et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat. Commun. 7, 12780 (2016).
Wu, N. C. et al. Different genetic barriers for resistance to HA stem antibodies in influenza H3 and H1 viruses. Science 368, 1335–1340 (2020).
Tan, G. S. et al. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 86, 6179–6188 (2012).
Yassine, H. M. et al. Use of hemagglutinin stem probes demonstrate prevalence of broadly reactive group 1 influenza antibodies in human sera. Sci. Rep. 8, 8628 (2018).
Lingwood, D. et al. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 489, 566–570 (2012).
Cotter, C. R., Jin, H. & Chen, Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog. 10, e1003831 (2014).
Sui, J. et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 265–273 (2009).
Vanderlinden, E. et al. Novel inhibitors of influenza virus fusion: structure–activity relationship and interaction with the viral hemagglutinin. J. Virol. 84, 4277–4288 (2010).
Hai, R. et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86, 5774–5781 (2012).
Shlomchik, M. J., Aucoin, A. H., Pisetsky, D. S. & Weigert, M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc. Natl Acad. Sci. USA 84, 9150–9154 (1987).
Doud, M. B. & Bloom, J. D. Accurate measurement of the effects of all amino-acid mutations on influenza hemagglutinin. Viruses 8, 155 (2016).
Doud, M. B., Hensley, S. E. & Bloom, J. D. Complete mapping of viral escape from neutralizing antibodies. PLoS Pathog. 13, e1006271 (2017).
Bloom, J. D. Software for the analysis and visualization of deep mutational scanning data. BMC Bioinformatics 16, 168 (2015).
Henry Dunand, C. J. et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J. Clin. Invest. 125, 1255–1268 (2015).
Chen, Y. Q. et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173, 417–429.e10 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Weitzner, B. D. et al. Modeling and docking of antibody structures with Rosetta. Nat. Protoc. 12, 401–416 (2017).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Li, L. et al. Improved integration of single-cell transcriptome and surface protein expression by LinQ-View. Cell Rep. Methods 1, 100056 (2021).
Zhuang, Q. et al. Diversity and distribution of type A influenza viruses: an updated panorama analysis based on protein sequences. Virol. J. 16, 85 (2019).
Burke, D. F. & Smith, D. J. A recommended numbering scheme for influenza A HA subtypes. PLoS ONE 9, e112302 (2014).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Li, L. et al. Librator, a platform for optimized sequence editing, design, and expression of influenza virus proteins. Preprint at bioRxiv https://doi.org/10.1101/2021.04.29.441999 (2021).
Weitzner, B. D., Kuroda, D., Marze, N., Xu, J. & Gray, J. J. Blind prediction performance of RosettaAntibody 3.0: grafting, relaxation, kinematic loop modeling, and full CDR optimization. Proteins 82, 1611–1623 (2014).
Stein, A. & Kortemme, T. Improvements to robotics-inspired conformational sampling in Rosetta. PLoS ONE 8, e63090 (2013).
Labute, P. Protonate3D: assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 75, 187–205 (2009).
Molecular Operating Environment (MOE). (2020).
Hub, J. S., de Groot, B. L., Grubmuller, H. & Groenhof, G. Quantifying artifacts in Ewald simulations of inhomogeneous systems with a net charge. J. Chem. Theory Comput. 10, 381–390 (2014).
Roe, D. R. & Cheatham, T. E. 3rd PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Case, D. A. et al. AMBER 2020 (University of California, San Francisco, 2020).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Wallnoefer, H. G., Liedl, K. R. & Fox, T. A challenging system: free energy prediction for factor Xa. J. Comput. Chem. 32, 1743–1752 (2011).
Barducci, A., Bonomi, M. & Parrinello, M. Metadynamics. WIREs Computat. Mol. Sci. 1, 826–843 (2011).
Barducci, A., Bussi, G. & Parrinello, M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 100, 020603 (2008).
Biswas, M., Lickert, B. & Stock, G. Metadynamics enhanced Markov modeling of protein dynamics. J. Phys. Chem. B 122, 5508–5514 (2018).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C., Bussi, G. PLUMED 2: new feathers for an old bird. Comp. Phys. Commun. 185, 604–613 (2014).
Ilott, A. J., Palucha, S., Hodgkinson, P. & Wilson, M. R. Well-tempered metadynamics as a tool for characterizing multi-component, crystalline molecular machines. J. Phys. Chem. B 117, 12286–12295 (2013).
Ramachandran, G. N., Ramakrishnan, C. & Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 7, 95–99 (1963).
Fernandez-Quintero, M. L. et al. Antibodies exhibit multiple paratope states influencing VH-VL domain orientations. Commun. Biol. 3, 589 (2020).
Salomon-Ferrer, R., Gotz, A. W., Poole, D., Le Grand, S. & Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Shao, J., Tanner, S. W., Thompson, N. & Cheatham, T. E. Clustering molecular dynamics trajectories: 1. Characterizing the performance of different clustering algorithms. J. Chem. Theory Comput. 3, 2312–2334 (2007).
Miyamoto, S. & Kollman, P. A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Adelman, S. A. & Doll, J. D. Generalized Langevin equation approach for atom/solid‐surface scattering: general formulation for classical scattering off harmonic solids. J. Chem. Phys. 64, 2375–2388 (1976).
Scherer, M. K. et al. PyEMMA 2: a software package for estimation, validation, and analysis of Markov models. J. Chem. Theory Comput. 11, 5525–5542 (2015).
Chodera, J. D. & Noe, F. Markov state models of biomolecular conformational dynamics. Curr. Opin. Struct. Biol. 25, 135–144 (2014).
Acknowledgements
We thank all of the volunteers who participated in this study; S. Andrews, R. Ahmed, J. Wrammert and K. Neu for initiating studies on the 2009 MIV, 2010 TIV and 2014 QIV cohorts; C. Mariottini, J. Feser, D. Stadlbauer and A.-K. Palm for their help on the cHA vaccine trial; I. Wilson for fruitful discussion and feedback on experimental design; B. Anderson and H. Turner for assistance with electron microscopy; and the teams at PATH, GSK, Cincinnati Children’s Hospital Medical Center, and Duke University for their work on the cHA vaccine trial (NCT03300050). This project was funded in part by the National Institute of Allergy and Infectious Diseases (NIAID; National Institutes of Health grant numbers K99AI159136 (to J.J.G.), U19AI082724 (to P.C.W.), U19AI109946 (to P.C.W.), U19AI057266 (to P.C.W.), P01AI097092 (to P.P.), R01AI145870-01 (to P.P.), R21AI146529 (to L.C.) and T32AI007244-36 (to J.H.), the NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) grant number HHSN272201400005C (to P.C.W.), HHSN272201400008C (to L.C., F.K., A.G.-S. and P.P.), and the NIAID Centers of Excellence for Influenza Research and Response (CEIRR) grant number 75N93019R00028 (to P.C.W., F.K., A.G.-S. and P.P.). This work was also partially supported by the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC; 75N93019C00051, to F.K., A.G.-S., P.P., A.B.W. and P.C.W.). The cHA vaccine trial and evaluation of immunity thereafter was funded in part by the Bill and Melinda Gates Foundation (OPP1084518). The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Gates Foundation. This study was also funded by the Austrian Science Fund grant number P34518 (to M.L.F.-Q. and K.R.L.).
Author information
Authors and Affiliations
Contributions
J.J.G., J.H., A.B.W. and P.C.W. conceptualized the study. J.J.G., J.H., H.A.U., L.L., L.Y.-L.L., A.W.F., M.L.F.-Q., C.H., C.T.S., M.M., G.O., F.A., L.G., N.-Y.Z., S.S., M.H., J.D.B. and L.C. developed the methodology. J.J.G., J.H., H.A.U., L.L., L.Y.-L.L., A.W.F., M.L.F.-Q., C.H., C.T.S., M.M., G.O., V.R., F.A., O.S., L.G., H.L.D., S.T.R., A.T.d.l.P., M.E.T., D.J.B., S.C. and L.C. conducted the investigation. J.J.G., J.H., H.A.U., L.L., M.L.F.-Q., M.M., L.G. and S.T.R. performed visualization. J.J.G., J.H., A.G.-S., P.P., F.K., L.C., A.B.W. and P.C.W. acquired funding. Project administration was done by J.J.G., J.H., C.H., A.G.-S., K.R.L., R.N., P.P., F.K., A.B.W. and P.C.W. J.J.G., J.H., A.B.W. and P.C.W. supervised the study. J.J.G. and J.H. wrote the original draft of the manuscript. J.J.G., J.H., H.A.U., L.L., L.Y.-L.L., C.H., C.T.S., M.M., G.O., A.W.F., O.S., L.G., H.L.D., N.-Y.Z., S.T.R., A.T.d.l.P., M.E.T., D.J.B., S.C., S.S., M.H., A.G.-S., R.N., P.P., J.D.B., F.K., L.C., A.B.W. and P.C.W. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The Icahn School of Medicine at Mount Sinai has patents (10736956, 10583188, 10137189, 10131695, 9968670, 9371366) and has submitted patent applications (10736956, 10583188, 10137189, 10131695, 9968670, 9371366) on universal influenza virus vaccines naming A.G.-S., R.N., P.P. and F.K. as inventors. The University of Chicago has submitted patent applications (632292804) on anti-anchor mAbs naming J.J.G. and P.C.W. as inventors. The A.G.-S. laboratory has received research support from Pfizer, Senhwa Biosciences, Kenall Manufacturing, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC and Merck, outside of the reported work. A.G.-S. has consulting agreements for the following companies involving cash and/or stock: Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Vaxalto, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories and Pfizer, outside of the reported work.
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Binding and neutralization features of anchor epitope-binding mAbs. Related to Fig. 1.
a, Proportion of HA+ mAbs binding to distinct HA domains (left) and proportion of stalk-binding mAbs binding the CS domain (right). Number in the center of the pie graphs represent the number of mAbs tested. b, Proportion of mAbs per cohort that bind the HA stalk domain. c, Negative stain 2D class averages of 047-09 4F04, 241 IgA 2F04, and 222-1C06 binding to H1 (A/California/7/2009 E376K HA). Imaging of 047-09 4F04 was performed at ×52,000 normal magnification and of 222-1C06 and 241 IgA 2F04 at ×62,000 normal magnification. d, Overlay of 047-09 4F04, 241 IgA 2F04, 222-1C06, and FISW84 (PDB:6HJQ) Fabs binding the anchor epitope of A/California/4/2009 HA. e, Overlay of CR8020 (PDB:3SDY), CR8043 (PDB:4NM8), and FISW84 (PDB:6HJQ) modeled on A/California/7/2009 E376G (PDB:4M4Y). f, Footprints of anchor mAb 222-1C06 on H1 (top; PDB: 4M4Y) and CR8020 and CR8043 on H3 (bottom; PDB:4WE4). g, Heatmap of apparent affinity (Kd; M) of anchor-targeting mAbs binding to historical and recent H1N1 viruses. h, Neutralization potency of anchor-binding mAbs (n = 15) against H1-expressing viruses. i, Representative microneutralization curves of anchor- (n = 42) and CS-binding (n = 29) mAbs against A/California/7/2009. j, IC80 of anchor- and CS-binding mAbs against A/California/7/2009. k, ADCC activity of mAbs targeting the CS and anchor epitopes. Dashed line represents the limit of detection (L.O.D). l, ADCC potency of mAbs targeting the anchor (n = 18 mAbs) and CS (n = 8 mAbs) epitopes. Data in h, j, and l are represented as mean ± S.D. Data in j and l were analyzed using a two-tailed unpaired non-parametric Mann-Whitney test.
Extended Data Fig. 2 Anchor-targeting mAb binding to influenza subtype, viral mutants, and polyreactivity antigen panel. Related to Fig. 1.
a, Proportion of anchor- (n = 50 mAbs) and CS-targeting mAbs (n = 37) binding influenza B viruses and H3N2 viruses. b, Negative stain 2D class averages (×62,000 normal magnification) of 222-1C06 binding to H2 (A/Singapore/1/1957), and H5 (A/Indonesia/5/2005). c, H2N2 neutralizing data of anchor- (n = 11 mAbs) and CS-binding mAbs (n = 4) represented as minimum neutralizing concentration. The limit of detection (L.O.D.) is 30 mg/ml. d, Proportion of mAbs targeting the anchor (n = 50 mAbs) or CS (n = 50 mAbs) epitope that are polyreactive. e, LPS binding strength, represented as area under the curve (AUC), of polyreactive mAbs targeting the anchor (n = 30 mAbs) and central stalk (n = 43 mAbs) epitopes. Data are mean ± S.D. f, g, Anchor- and CS epitope-binding mAbs were tested for binding to A/California/7/2009 HA with naturally occurring and experimentally determined mutations induced by 045-09 2B06, a CS-binding mAb. f, Location of mutations modeled on A/California/4/2009 HA (PDB: 4JTV). Residues in blue are located on HA1 and residues in red are located on HA2. Outlines represent binding footprints of 047-09 4F04 (sky blue) and CR9114 (green). g, Heatmap of mAb binding to WT and mutant HAs shown as the proportion of signal relative to mAb binding to the WT HA. Data in a and d were analyzed using Fisher’s Exact tests. Data in e were analyzed using a two-tailed unpaired non-parametric Mann-Whitney test.
Extended Data Fig. 3 Anchor epitope-targeting mAbs are potently protective in vivo and lack ADCC activity. Related to Fig. 1.
a, b, Mice were prophylactically (2 h prior to infection; a) or therapeutically (48 h after infection; b) administered i.p. a cocktail of mAbs (n = 5 mAbs/cocktail) against the anchor- or CS-, or an anthrax-specific antibody. Mice were infected with 10 LD50 of A/Netherlands/602/2009 H1N1. Weight loss (top) and survival (bottom) of mice in each treatment group. c, Lung viral titers of mice in each prophylactic treatment group infected with 1 LD50 of A/Netherlands/602/2009. dpi, days post infection. d, Mice were prophylactically (2 h prior to infection) administered i.p. a cocktail of mAbs (n = 5 mAbs/cocktail) against the anchor- or CS-, or an anthrax-specific antibody. Mice were infected with 10 LD50 of A/Fort Monmouth/1/1947 H1N1. Weight loss (top) and survival (bottom) of mice in each treatment group. For a, b, and d, 10 mice per treatment group were used and data are pooled from two independent experiments. For c, 5 mice per treatment group and timepoint were used except for anchor cocktail group at dpi 3 only 4 mice were used. Data in a, b, and d are represented as mean ± S.D and data in c are represented by mean ± S.E.M. Kaplan-Meier curves in a, b, d were analyzed using a Log-rank Mantel-Cox test, and data in c were analyzed using multiple two-tailed unpaired non-parametric Kruskal-Wallis tests.
Extended Data Fig. 4 Additional repertoire and structural features of mAbs binding the anchor epitope. Related to Fig. 2.
a, VH locus usage by anchor- (n = 52 mAbs) and CS-binding mAbs (n = 37 mAbs). b, VH1 gene usage of mAbs targeting the CS epitope. c, VK locus usage by anchor- (n = 52 mAbs) and CS-binding mAbs (n = 37 mAbs). d, JK gene usage by anchor epitope-binding mAbs. e, Clonal expansions of anchor epitope-targeting mAbs. Numbers indicate heavy and light chain parings, which are described in Extended Data Table 2. f, Heavy and light chain sequences of the public clone. g, h, Mutations (g) and CDR3 amino acid (AA) lengths (h) of heavy and light chains of mAbs binding the anchor (n = 52 mabs) or CS (n = 37 mAbs) epitopes. Data are mean ± S.D. i, Cryo-EM map of 222-1C06 binding to A/California/7/2009 E376K HA. j, k, Local resolution (j) and Fourier Shell Correlation (k) of 222-1C06 binding to HA. l, Aromatic pockets of 222-1C06 binding A/California/7/2009 E376K and FISW84 binding to A/duck/Alberta/35/1976 (PDB:6HJQ; top) and overlay of epitope:paratope interaction (bottom). m, MD simulations demonstrating the K-CDR3 NWP and H-CDR2 Y58 motifs of 222-1C06, FISW84, 241 IgA 2F04, and SFV009 3G01 binding to HA A/California/7/2009 HA. For left-hand panels in l and all panels in m, HA epitope contact residues (maroon) and heavy chain (green) and light chain (yellow) antibody contact residues of anchor mAb paratopes. Peach highlighted amino acids represent the fusion peptide of HA2. n, Fab-Fab interactions of the aromatic pocket of 222-1C06. o, MD simulation of the paratope flexibility of 222-1C06, highlighting the p-stacking of H-CDR2 and K-CDR3. p, Conservation of side-chain contacts of 222-1C06 across seasonal human H1N1 viruses circulating between 1918-2019. q, Deep mutational scanning of the side-chain contacts of 222-1C06. Data in a and c were analyzed using a Chi-square test, and data in g, h were analyzed by two-tailed unpaired non-parametric Mann-Whitney tests.
Extended Data Fig. 5 Features of anchor-targeting MBCs and EMPEM 2D classes. Related to Fig. 3.
a, 33 mAbs with anchor epitope-binding mAb repertoire features were generated and tested for competing for binding with 047-09 4F04. b, c, Number of heavy chain mutations (b) and isotype usage (c) of B cells with repertoire features of anchor-binding mAbs (n = 119 cells) or utilize VH1-69/kappa (n = 365 cells). d, e, 2D class averages of pAbs from donors 236 (d) and 241 (e) at days 7 and 14 post immunization binding to A/Michigan/45/2015 HA (×62,000 normal magnification). The last row of 2D classes in d is HA monomer complexes processed independently from trimer complexes. Data in b are represented as mean ± S.D. Data in b were analyzed using a two-tailed paired non-parametric Wilcoxon matched-pairs signed rank test.
Extended Data Fig. 6 Serum antibody kinetics of anchor- and CS-epitope binding antibodies after cHA vaccination and mAb binding to recombinant HAs. Related to Fig. 4.
a, b, EC50s of serum antibodies competing for binding with 047-09 4F04 for binding to the anchor epitope (a) and CR9114 for binding to the CS epitope (b). a, b, Kinetics of serum antibody responses against the anchor (a) and CS (b) epitopes. Data are mean + S.D. c, d, Proportion of stalk+ mAbs per donor (c) or proportion of donors with an isolated anchor mAb (d) upon first exposure to the pH1N1 virus (2009 MIV cohort) relative to donors who have repeatedly been exposed to pH1N1 (2010 TIV and 2014 QIV). Data in c are mean ± S.D. Data in c includes only donors with an isolated anti-stalk mAb, whereas d includes all donors. e, f, Antibody titers (EC50) of serum antibodies collected on day 113 and day 420 against the anchor epitope (e) and the CS epitope (f). Lines connect titers from the same donor and each pair of symbols represents one donor. g, Proportion of anchor epitope-binding mAbs binding to cHA (cH6/1) or mini-HA (n = 50). h, Representative flow cytometry plots of mAbs binding to A/California/7/2009 Cal09 HA and mini-HA (left) and geometric mean fluorescence intensity (gMFI) of mAbs binding to Cal09 and mini-HA (right). Data represent the mean ± S.D. and each symbol represents an individual mAb. i, Proportion of anchor epitope-targeting mAbs binding to A/California/7/2009 recombinant HA with a GCN4 or fibritin trimerization domain (n = 50). For data in a, b, e, and f, Group 1 n = 10 participants, group 2 n = 7 participants, group 4 n = 7 participants, group 3&5 n = 6. For data in c, first exposure n = 7 donors and repeated exposure n = 4 donors. For data in d, first exposure n = 10 donors and repeated exposure n = 13 donors. Data in a, b were analyzed using a two-tailed two-way ANOVA testing for simple effects within rows, data in c and h were analyzed using a two-tailed unpaired non-parametric Mann-Whitney test, data in d, g, and i were analyzed using Fisher’s Exact test, and data in c, d were analyzed using a two-tailed paired non-parametric Wilcoxon matched-pairs signed rank test. See also Supplementary Fig. 1 for gating strategy for panel h.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 and Supplementary Tables 1–6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guthmiller, J.J., Han, J., Utset, H.A. et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 602, 314–320 (2022). https://doi.org/10.1038/s41586-021-04356-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04356-8
This article is cited by
-
Scientific highlights of the 9th ESWI Influenza Conference
One Health Outlook (2024)
-
Applying valency-based immuno-selection to generate broadly cross-reactive antibodies against influenza hemagglutinins
Nature Communications (2024)
-
Triple tandem trimer immunogens for HIV-1 and influenza nucleic acid-based vaccines
npj Vaccines (2024)
-
Inadequate structural constraint on Fab approach rather than paratope elicitation limits HIV-1 MPER vaccine utility
Nature Communications (2023)
-
Co-evolution of immunity and seasonal influenza viruses
Nature Reviews Microbiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.