Abstract
Pro-inflammatory T cells in the central nervous system (CNS) are causally associated with multiple demyelinating and neurodegenerative diseases1,2,3,4,5,6, but the pathways that control these responses remain unclear. Here we define a population of inflammatory group 3 innate lymphoid cells (ILC3s) that infiltrate the CNS in a mouse model of multiple sclerosis. These ILC3s are derived from the circulation, localize in proximity to infiltrating T cells in the CNS, function as antigen-presenting cells that restimulate myelin-specific T cells, and are increased in individuals with multiple sclerosis. Notably, antigen presentation by inflammatory ILC3s is required to promote T cell responses in the CNS and the development of multiple-sclerosis-like disease in mouse models. By contrast, conventional and tissue-resident ILC3s in the periphery do not appear to contribute to disease induction, but instead limit autoimmune T cell responses and prevent multiple-sclerosis-like disease when experimentally targeted to present myelin antigen. Collectively, our data define a population of inflammatory ILC3s that is essential for directly promoting T-cell-dependent neuroinflammation in the CNS and reveal the potential of harnessing peripheral tissue-resident ILC3s for the prevention of autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
ILC3 RNA-sequencing data are available at the Gene Expression Omnibus under accession number GSE165580. Faecal 16S data are deposited at the NCBI Sequence Read Archive under BioProject accession number PRJNA761714. Source data are provided with this paper.
References
Ota, K. et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346, 183–187 (1990).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112, 415–422 (2003).
Sulzer, D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017).
Lindestam Arlehamn, C. S. et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 11, 1875 (2020).
Lincoln, M. R. et al. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat. Genet. 37, 1108–1112 (2005).
Hamza, T. H. et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 42, 781–785 (2010).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993 (2014).
Fallis, R. J., Raine, C. S. & McFarlin, D. E. Chronic relapsing experimental allergic encephalomyelitis in SJL mice following the adoptive transfer of an epitope-specific T cell line. J. Neuroimmunol. 22, 93–105 (1989).
Brochard, V. et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 119, 182–192 (2009).
Browne, T. C. et al. IFN-γ production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. 190, 2241–2251 (2013).
Lodygin, D. et al. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature 566, 503–508 (2019).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Sonnenberg, G. F. & Hepworth, M. R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 19, 599–613 (2019).
Mair, F. & Becher, B. Thy1+ Sca1+ innate lymphoid cells infiltrate the CNS during autoimmune inflammation, but do not contribute to disease development. Eur. J. Immunol. 44, 37–45 (2014).
Hatfield, J. K. & Brown, M. A. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell. Immunol. 297, 69–79 (2015).
Kwong, B. et al. T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat. Immunol. 18, 1117–1127 (2017).
Yamano, T. et al. Aire-expressing ILC3-like cells in the lymph node display potent APC features. J. Exp. Med. 216, 1027–1037 (2019).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Takeshita, Y. & Ransohoff, R. M. Inflammatory cell trafficking across the blood–brain barrier: chemokine regulation and in vitro models. Immunol. Rev. 248, 228–239 (2012).
Perry, J. S. et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci. Transl. Med. 4, 145ra106 (2012).
Lin, Y. C. et al. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann. Clin. Transl. Neurol. 2, 445–455 (2015).
Degn, M. et al. Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult. Scler. 22, 1013–1020 (2016).
Serafini, B. et al. RORγt expression and lymphoid neogenesis in the brain of patients with secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 75, 877–888 (2016).
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Hepworth, M. R. et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
von Burg, N. et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc. Natl Acad. Sci. USA 111, 12835–12840 (2014).
Ting, J. P. & Trowsdale, J. Genetic control of MHC class II expression. Cell 109, S21–S33, (2002).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Bryant, P. W., Lennon-Dumenil, A. M., Fiebiger, E., Lagaudriere-Gesbert, C. & Ploegh, H. L. Proteolysis and antigen presentation by MHC class II molecules. Adv. Immunol. 80, 71–114 (2002).
Zhang, Q. & Vignali, D. A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44, 1034–1051 (2016).
Lee, J. Y. et al. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell 180, 79–91 (2020).
Koda, T. et al. Sema4A is implicated in the acceleration of Th17 cell-mediated neuroinflammation in the effector phase. J. Neuroinflammation 17, 82 (2020).
Hur, E. M. et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 8, 74–83 (2007).
Giles, D. A., Duncker, P. C., Wilkinson, N. M., Washnock-Schmid, J. M. & Segal, B. M. CNS-resident classical DCs play a critical role in CNS autoimmune disease. J. Clin. Invest. 128, 5322–5334 (2018).
Mundt, S. et al. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 4, eaau8380 (2019).
Korn, T. & Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 17, 179–194 (2017).
Waisman, A. & Johann, L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J. Mol. Med. 96, 1279–1292 (2018).
Frommer, F. et al. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J. Immunol. 181, 5748–5759 (2008).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119 (2018).
Montaldo, E. et al. Human RORγt+CD34+ cells are lineage-specified progenitors of group 3 RORγt+ innate lymphoid cells. Immunity 41, 988–1000 (2014).
Lim, A. I. et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168, 1086–1100 (2017).
Scoville, S. D. et al. A progenitor cell expressing transcription factor RORγt generates all human innate lymphoid cell subsets. Immunity 44, 1140–1150 (2016).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Hashimoto, K., Joshi, S. K. & Koni, P. A. A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis 32, 152–153 (2002).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol 1, 4 (2001).
Lee, P. P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Dobes, J. et al. A novel conditional Aire allele enables cell-specific ablation of the immune tolerance regulator Aire. Eur. J. Immunol. 48, 546–548 (2018).
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Lochner, M. et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγ t+ T cells. J. Exp. Med. 205, 1381–1393 (2008).
Croxford, A. L., Kurschus, F. C. & Waisman, A. Cutting edge: an IL-17F-CreEYFP reporter mouse allows fate mapping of Th17 cells. J. Immunol. 182, 1237–1241 (2009).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Miller, S. D., Karpus, W. J. & Davidson, T. S. Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 88, 15.1.1–15.1.20 (2010).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Kamran, P. et al. Parabiosis in mice: a detailed protocol. J. Vis. Exp. 80, e50556 (2013).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Edgar, R. C. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. Preprint at https://doi.org/10.1101/074161 (2016).
Cole, J. R. et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642 (2014).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Louveau, A., Filiano, A. J. & Kipnis, J. Meningeal whole mount preparation and characterization of neural cells by flow cytometry. Curr. Protoc. Immunol. 121, e50 (2018).
Acknowledgements
We thank members of the Sonnenberg laboratory for discussions and critical reading of the manuscript; the Microbiome Core and Epigenomics Core of Weill Cornell Medicine and G. G. Putzel for technical assistance; C. Gai of Weill Cornell’s Center of Comparative Medicine and Pathology (CCMP) for performing the parabiosis surgeries; and I. Ivanov of Columbia University for sharing mouse lines. The Sonnenberg laboratory is supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, R01AI162936, R21CA249284 and U01AI095608), the National Institute of Allergy and Infectious Diseases (NIAID) Mucosal Immunology Studies Team (MIST), the Crohn’s and Colitis Foundation, the Searle Scholars Program, the American Asthma Foundation Scholar Award, Pilot Project Funding from the Center for Advanced Digestive Care (CADC), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, a Wade F.B. Thompson–Cancer Research Institute (CRI) CLIP Investigator grant, the Meyer Cancer Center Collaborative Research Initiative, the Dalton Family Foundation, L. and G. Greenberg, and the Jill Roberts Institute for Research in Inflammatory Bowel Disease. G.F.S. is a CRI Lloyd J. Old STAR. J.B.G is supported by the NIAID of the National Institutes of Health under award number F31AI138389-01A1; A.M.J is supported by T32DK116970; and the Waisman laboratory is supported by the Deutsche Forschungsgemeinschaft (DFG) grants AW1600/10-1, AW1600/11-1 and AW1600/14-1, as well as by the National Multiple Sclerosis Society (NMSS) grant RG 1707-28780.
Author information
Authors and Affiliations
Contributions
J.B.G. and G.F.S. conceived the project. J.B.G. performed most of the experiments and analysed the data. A.S., T.R., A.A. and A.M.J. also helped to process tissues, generated experimental datasets and analysed data from EAE in IiMOG mice (A.S. and T.R.) or Rorccre mice (A.A. and A.M.J.). C.N.P. processed tissues and generated the immunofluorescence data. M.M. coordinated the selection and T.V. provided and supervised the selection and analysis of samples from patients with RRMS, as well as providing scientific discussion. G.E., K.G. and A.D. provided essential mouse models, scientific advice and expertise. A.W. jointly supervised the research, provided essential mouse strains and contributed to the formation of the project. J.B.G. and G.F.S. wrote the manuscript, with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
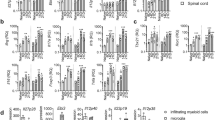
Extended Data Fig. 1 Characterization of ILC3s in the CNS.
a, Representative time course and clinical disease categorization of active EAE (n = 4, 5 mice/timepoint). b-c, Quantification of ILC3 frequency and absolute counts within indicated tissues at steady state (Naive) (n = 6 mice) versus EAE onset (d11, n = 7), acute (d15, n = 8) or chronic (d20, n = 9) phase (b) and reverse flow cytometry gating strategy defining all GFP+ cells in the CNS (d15) (n = 4 mice/group) (c) during EAE in Rorc-eGFP mice. d-e, Naive C57BL/6 mice (n = 4 mice/group) were immunized with either PBS (Naive), CFA/PTx alone, or with CFA/PTx/MOGp. At day 15 post-immunization, ILC3s in the CNS, inLN and cLN were enumerated by flow cytometry: d, Representative flow cytometry gating strategy for ILC3s in CNS (Lin1 = CD3, CD5, CD8, Lin2 = CD11b, CD11c, B220), e, Quantitation of frequencies and absolute counts of ILC3s. f-g, Representative flow cytometry on YFP+ ILCs (f) and quantification of differential expression of ILC heterogeneity in YFP+/- ILCs (g) in the CNS of Rorc-creeYFP mice during active EAE (n = 6 mice/group) (Lin 1 = B220, CD11b, CD11c, Lin 2 = CD3ε, CD5, CD8, Ly6C). Data in a-f are representative of two independent experiments with similar results and data in b are pooled from two independent experiments. Results are shown as mean ± s.d. Statistics are calculated by one-way (b, e) analysis of variance (ANOVA) with Sidak’s multiple comparisons test.
Extended Data Fig. 2 Phenotypic analyses of ILC3s in the CNS and peripheral blood.
a-c, Heat maps showing absolute log normalized counts (one plus log 2) (a, b) or relative expression Z-scores (c) from RNA sequencing of indicated lineage-specifying genes in sorted ILC3s from the cLN, CNS, or small intestine lamina propria (SI-LP) of Rorc-eGFP mice during peak of active EAE (n = 4 mice). d, Quantification of cytokine production by ILC3s (CD45+, CD3ε-, CD5-, CD8α-, TCRγδ-, NK1.1-, CD11b-, CD11c-, B220-, CD127+, CD90.2+, KLRG1-, RORγt+) in indicated tissues during EAE (day 15) C57BL/6 mice (n = 4 mice). e, Representative flow cytometry gating strategy to detect ILC3s in human PBMCs (Lineage = CD19, CD94, CD14, CD123, FcR1a, CD11c) and frequency quantification of ILC3s in healthy control (HC) or RRMS samples (n = 18 sample-pairs – Supplementary Table 1). Data in d are representative of two independent experiments with similar results. Data in e are pooled and representative of two independent flow cytometry experiments on cryopreserved PBMC sample sets with similar results. Results are shown as mean ± s.d. Statistics are calculated by one-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test (d).
Extended Data Fig. 3 Expression of MHCII and co-stimulatory molecules on ILC3s during neuroinflammation.
a, d, Representative histograms and quantification of MHCII (a), and CD80 or CD86 (d) expression on ILC3s in indicated tissues of Rorc-eGFP mice at steady state (Naive) (n = 3 mice) versus EAE onset (d11, n = 4), acute (d15, n = 4) or chronic (d20, n = 5) phase. b, Naive C57BL/6 mice were immunized with either PBS (Naive), CFA/PTx or with CFA/PTx/MOGp (n = 4 mice/group). At day 15 EAE, frequency and counts of MHCII+ ILC3s in the CNS and cLN were enumerated by flow cytometry. c, e Representative staining and quantification of HLA-DR (c) and co-stimulatory molecules (e) on human blood ILC3s (n = 18 samples/group). Data in a, b, d are representative of two independent experiments with similar results. Results are shown as mean ± s.d. Statistics are calculated by one-way (b) analysis of variance (ANOVA) with Sidak’s multiple comparisons test.
Extended Data Fig. 4 ILC3s in the periphery do not express co-stimulatory molecules.
a-b, Naive C57BL/6 mice were immunized with either PBS (Naive), CFA/PTx or with CFA/PTx/MOGp (EAE) (n = 4 mice/group). At day 15 post immunization, expression of co-stimulatory molecules CD80, CD86 (a) and OX40L, CD40, and CD30L (b) by MHCII+ ILC3s in the mLN, inLN, cLN and CNS were enumerated by flow cytometry. Data are representative of three independent experiments with similar results. Results are shown as mean ± s.d.
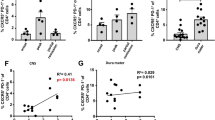
Extended Data Fig. 5 ILC3s are found in the cerebral spinal fluid of patients with RRMS and express HLA-DR and CD86.
a, Validation of anti-RORγt antibody staining on single cell suspensions from human donor tonsils compared to FMO (Fluorescence Minus One) control indicating staining without anti-RORγt antibody. b-e, Cerebral spinal fluid (CSF) was obtained from 7 patients with RRMS and one control individual (other neurological disease, OND). Individuals were further stratified by the presence or absence of contrast enhancing lesions (CEL) as well as CEL number (Supplementary Table 2). CSF was processed immediately by centrifuging for 10 min at 400 x g and staining for ILC3s as indicated (b) (Lineage = CD19, CD94, CD14, CD123, FcR1a, CD11c). Gated RORγt+ populations in the indicated tissues exhibited dim staining for CD45, which is a defining feature of ILC3s, relative to CD4 T cells (c). Indicated frequencies of ILC3s in the CSF were quantified (d). PBMCs were used as controls during each collection and used for comparison of HLA-DR or CD86 expression on ILC3s in the CSF (e). Results are shown as mean (d) and RRMS flow cytometry is representative flow cytometry from CSF of patient #2 (Supplementary Table 2). Human tonsil data is representative of 3 individual tonsil samples.
Extended Data Fig. 6 Interrogation of ILC3 and T cell interactions in the CNS.
a, Representative immunofluorescence staining of fixed dura meninges, brain cerebellum and spinal cord (edge denoted by dashed white line) from Rorc-eGFP mice during day 11 or day 18 of active EAE showing enrichment of GFP+ cells in focal lesions of the parenchyma (representative of n = 3 mice/timepoint). b-c, Frequency of cytokine producing 2D2 T cells after 72 h co-culture with ex vivo sorted cDC or alone (2D2 T cells alone) from the CNS (b) or the mLN (c) (pooled from 5 mice, d18 EAE) in the presence of MOGp +/- α-MHCII blocking antibody. d, To determine the ability to process full-length antigen 2D2 T cells and ILC3s were sorted from the CNS (pooled from n = 5 mice, d19 EAE) and co-cultured as indicated for 72 h prior to determination of cell counts, staining of IFNγ or CD25 for flow cytometry. Resulting 2D2 cell counts were measured and normalized as a fold-change in comparison to myelin-specific 2D2 T cells cultured alone (no APC) and treated with MOGp1-125 (dashed line) (left graph). Data in d are pooled from two independent experiments and data are representative of two (b-c) independent experiments with similar results. Data were necessarily pooled in noted experiments due to limited cell numbers. Results are shown as mean ± s.d. Statistics are calculated by one-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test. Data points indicate technical well replicates and dashed lines (b-d) indicate baseline cytokine production by 2D2 T cells alone from indicated tissues.
Extended Data Fig. 7 Selective targeting of T cells and ILC3s through RORγt.
a, Expression of MHCII was quantified by flow cytometry in indicated antigen-presenting cells from the cLN or CNS of indicated mice at steady state (n = 4 mice/group). b-c Representative flow cytometry gating strategy for indicated immune cell populations in the CNS (b) and expression of YFP in indicated cell populations and tissues (c) of Rorc-creeYFP mice at day 15 of active EAE. d, Reverse flow cytometry gating strategy defining all YFP+ fate-mapped cells in the CNS of Rorc-creeYFP mice (day 15 EAE) (Lin 1 = B220, CD11b, CD11c, Lin 2 = CD3ε, CD5, CD8, Ly6C). Results are shown as mean ± s.d. Statistics are calculated by one-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test (a). Data in a-d are representative of two independent experiments with similar results
Extended Data Fig. 8 Effect of ILC3-specific MHCII and generalized intestinal inflammation on neuroinflammation.
a-d, Naive 2D2 T cells (Thy1.1+) were transferred into recipient mice, which were immunized 24 h later to induce active EAE. At day 14 EAE, frequencies and counts (a), activation/proliferation (b), and polarization (c) of donor Thy1.1+ 2D2 T cells (c, upper panel) or endogenous T cells (c, lower panel) were analysed by intracellular flow cytometry in the cLN or inLN (n = 9 mice/group (a, b), n = 5 mice/group (c)). d, Frequencies of IFNγ-producing endogenous T cells in the CNS were quantified by intracellular flow cytometry cytokine staining (n = 8 mice/group). e-f, Passive EAE was induced in recipient mice and day 15 frequencies of Thy1.1+ 2D2 T cells in the cLN and CNS were determined by flow cytometry (n = 4 mice/group) (e). Representative H&E of fixed transverse spinal cord sections of mice shown at 4X magnification (top, scale bar = 300 µM) with 20X inset magnification (bottom, scale bar = 75 µm) of cellular infiltration near ventromedial fissure at day 15 passive EAE (f). g-h, Average clinical scores in indicated mice (MHCIIΔTcell = CD4-cre+ x H2-Ab1fl/fl) after induction of active EAE (n = 4 mice/group (g), n = 5 mice/group (h)). i, C57BL/6 mice were treated with either 3% D.S.S. in the drinking water for 7 days or were orally gavaged with C. rodentium (n = 5 mice/group). Active EAE was induced 14 days later and clinical scores were taken on mice or controls. Data are representative of two (g-i) or three (a-f) independent experiments with similar results. Data are pooled from two independent experiments in a, b, d. Results are shown as mean ± s.d. (a-e) or s.e.m (g-i) Statistics are calculated by two-way analysis of variance (ANOVA) (c, d) with Sidak’s multiple comparisons test or unpaired, two-tailed t-test (Mann–Whitney U-test) (a, b, e).
Extended Data Fig. 9 Targeting peripheral ILC3s and their role in neuroinflammation.
a-d, Littermate H2-Ab1fl/fl and MHCIIΔILC3 mice were treated with Vancomycin (Vanco) in the drinking water for 7 days prior to induction of active EAE and Vanco was continued throughout EAE. a, PCoA (Weighted UniFrac) of 16S rRNA gene sequencing of faecal samples at indicated treatment timepoints (n = 5 mice/group, rep. of N = 2). At the end of EAE plus vancomycin treatment, b, colon lengths, spleen mass, c, and total frequencies of endogenous neutrophils, CD4 T cells, and transferred Thy1.1+ 2D2 T cells in the indicated gastro-intestinal tissues. LI-LP = large intestine lamina propria. d, Daily (n = 5 mice/group) and cumulative clinical scores of mice (n = 9 mice/group, pooled from N = 2). e-f, Splenic antigen-presenting cells (APCs: DCs or B cells) were sort-purified from steady state (e) or CFA-treated mice (f, d10) and co-cultured with 2D2 T cells previously stained with violet cell trace violet. Groups included 2D2 T cells alone (no APC), 2D2 T cells plus APC, or 2D2 T cells plus APC and MOG peptide. After 72 h, proliferation of 2D2 T cells was determined by flow cytometry to measure cell trace violet dilution from cell division. g, Average clinical scores in indicated mice after induction of active EAE (n = 18-21 mice/group left panel pooled from three independent experiments with similar results, n = 13-4 mice/group middle panel pooled from three independent experiments with similar results, right panel n = 3 mice/group representative of two independent experiments with similar results). Results are shown as mean ± s.e.m (d left panel, g) or mean ± s.d. (a-c, d right panel). Statistics are calculated by two-tailed t-test (Mann–Whitney U-test) (b, d) or two-way analysis of variance (ANOVA) (c) with Sidak’s multiple comparisons test. Results in a are representative of two independent experiments (n = 4-5 mice/group) and data in b-d are pooled from N = 2.
Extended Data Fig. 10 Antigen presenting group 3 innate lymphoid cells orchestrate neuroinflammation.
a, ILC3 family heterogeneity with inflammatory ILC3s (iILC3s) depicted in yellow. b, During autoimmune neuroinflammation, iILC3s enter the CNS from the circulation and are essential to promote pro-inflammatory T cell responses and demyelinating disease through antigen presentation. c, Tissue-resident and peripheral ILC3s retain tolerogenic potential, and when targeted to express myelin peptide can eliminate self-specific T cells and prevent demyelinating disease.
Supplementary information
Supplementary Tables
Supplementary Table 1 contains the sex and age range of patients with RRMS who provided PBMC samples. Supplementary Table 2 provides information on the patients with RRMS.
Source data
Rights and permissions
About this article
Cite this article
Grigg, J.B., Shanmugavadivu, A., Regen, T. et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature 600, 707–712 (2021). https://doi.org/10.1038/s41586-021-04136-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04136-4
This article is cited by
-
Increased regulatory activity of intestinal innate lymphoid cells type 3 (ILC3) prevents experimental autoimmune encephalomyelitis severity
Journal of Neuroinflammation (2024)
-
Group 3 innate lymphoid cells in intestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
The emerging family of RORγt+ antigen-presenting cells
Nature Reviews Immunology (2024)
-
Exploring the global immune landscape of peripheral blood mononuclear cells in H5N6-infected patient with single-cell transcriptomics
BMC Medical Genomics (2023)
-
Identification of two migratory colon ILC2 populations differentially expressing IL-17A and IL-5/IL-13
Science China Life Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.