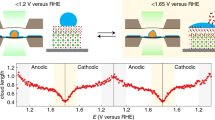
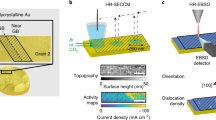
Abstract
Transition metal (oxy)hydroxides are promising electrocatalysts for the oxygen evolution reaction1,2,3. The properties of these materials evolve dynamically and heterogeneously4 with applied voltage through ion insertion redox reactions, converting materials that are inactive under open circuit conditions into active electrocatalysts during operation5. The catalytic state is thus inherently far from equilibrium, which complicates its direct observation. Here, using a suite of correlative operando scanning probe and X-ray microscopy techniques, we establish a link between the oxygen evolution activity and the local operational chemical, physical and electronic nanoscale structure of single-crystalline β-Co(OH)2 platelet particles. At pre-catalytic voltages, the particles swell to form an α-CoO2H1.5·0.5H2O-like structure—produced through hydroxide intercalation—in which the oxidation state of cobalt is +2.5. Upon increasing the voltage to drive oxygen evolution, interlayer water and protons de-intercalate to form contracted β-CoOOH particles that contain Co3+ species. Although these transformations manifest heterogeneously through the bulk of the particles, the electrochemical current is primarily restricted to their edge facets. The observed Tafel behaviour is correlated with the local concentration of Co3+ at these reactive edge sites, demonstrating the link between bulk ion-insertion and surface catalytic activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The experimental data that support the findings of this study are available in ref. 31.
References
Burke, M. S., Enman, L. J., Batchellor, A. S., Zou, S. & Boettcher, S. W. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chem. Mater. 27, 7549–7558 (2015).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Dau, H. et al. The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724–761 (2010).
Kuai, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal. 3, 743–753 (2020).
Mefford, J. T., Zhao, Z., Bajdich, M. & Chueh, W. C. Interpreting Tafel behavior of consecutive electrochemical reactions through combined thermodynamic and steady state microkinetic approaches. Energy Environ. Sci. 13, 622–634 (2020).
Mefford, J. T., Akbashev, A. R., Zhang, L. & Chueh, W. C. Electrochemical reactivity of faceted β-Co(OH)2 single crystal platelet particles in alkaline electrolytes. J. Phys. Chem. C 123, 18783–18794 (2019).
Doyle, A. D., Bajdich, M. & Vojvodic, A. Theoretical insights to bulk activity towards oxygen evolution in oxyhydroxides. Catal. Lett. 147, 1533–1539 (2017).
Burke, M. S., Kast, M. G., Trotochaud, L., Smith, A. M. & Boettcher, S. W. Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638–3648 (2015).
Wahab, O. J., Kang, M. & Unwin, P. R. Scanning electrochemical cell microscopy: A natural technique for single entity electrochemistry. Curr. Opin. Electrochem. 22, 120–128 (2020).
Bentley, C. L. et al. Nanoscale electrochemical mapping. Anal. Chem. 91, 84–108 (2019).
Bentley, C. L., Kang, M. & Unwin, P. R. Nanoscale structure dynamics within electrocatalytic materials. J. Am. Chem. Soc. 139, 16813–16821 (2017).
Bajdich, M., García-Mota, M., Vojvodic, A., Nørskov, J. K. & Bell, A. T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 135, 13521–13530 (2013).
Stevens, M. B. et al. Ternary Ni-Co-Fe oxyhydroxide oxygen evolution catalysts: Intrinsic activity trends, electrical conductivity, and electronic band structure. Nano Res. 12, 2288–2295 (2019).
Dette, C., Hurst, M. R., Deng, J., Nellist, M. R. & Boettcher, S. W. Structural evolution of metal (oxy)hydroxide nanosheets during the oxygen evolution reaction. ACS Appl. Mater. Interfaces 11, 5590–5594 (2019).
Toma, F. M. et al. Mechanistic insights into chemical and photochemical transformations of bismuth vanadate photoanodes. Nat. Commun. 7, 12012 (2016).
Kamachi, M. U. & Padhy, N. Electrochemical scanning probe microscope (EC-SPM) for the in situ corrosion study of materials: an overview with examples. Corros. Rev. 29, 73–103 (2011).
Deng, J. et al. Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy. Nano Lett. 17, 6922–6926 (2017).
Bernard, P. et al. AC quartz crystal microbalance applied to the studies of the nickel hydroxide behaviour in alkaline solutions. Electrochim. Acta 36, 743–746 (1991).
Young, M. J., Kiryutina, T., Bedford, N. M., Woehl, T. J. & Segre, C. U. Discovery of anion insertion electrochemistry in layered hydroxide nanomaterials. Sci. Rep. 9, 2462 (2019).
Li, F., Hillman, A. R., Lubetkin, S. D. & Roberts, D. J. Electrochemical quartz crystal microbalance studies of potentiodynamic electrolysis of aqueous chloride solution: surface processes and evolution of H2 and Cl2 gas bubbles. J. Electroanal. Chem. (Lausanne) 335, 345–362 (1992).
Cherepanova, S., Leont’eva, N., Drozdov, V. & Doronin, V. Thermal evolution of Mg-Al and Ni-Al layered double hydroxides: the structure of the dehydrated phase. Acta Crystallogr. A 72, 651–659 (2016).
Ma, R. et al. Tetrahedral Co(II) coordination in α-type cobalt hydroxide: Rietveld refinement and X-ray absorption spectroscopy. Inorg. Chem. 45, 3964–3969 (2006).
Hall, D. S., Lockwood, D. J., Bock, C. & MacDougall, B. R. Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proc. R. Soc. A 471, 20140792 (2015).
Liu, S., Li, L., Patterson, N. A. & Manthiram, A. Morphological transformations during in situ electrochemical generation of 2-dimensional Co3O4 hexagonal nanoplates. J. Electrochem. Soc. 163, A150–A155 (2016).
Lim, J. et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353, 566–571 (2016).
Gerken, J. B. et al. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 133, 14431–14442 (2011).
McAlpin, J. G. et al. EPR evidence for Co(iv) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 132, 6882–6883 (2010).
Risch, M. et al. Water oxidation by amorphous cobalt-based oxides: in situ tracking of redox transitions and mode of catalysis. Energy Environ. Sci. 8, 661–674 (2015).
Kanan, M. W. et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13692–13701 (2010).
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 1, 711–719 (2018).
Mefford, J. T. Replication data for "Correlative operando microscopy of oxygen evolution electrocatalysts". https://doi.org/10.7910/DVN/TNTHXS (2021).
Acknowledgements
J.T.M., A.R.A., W.E.G. and W.C.C. acknowledge funding provided by the US Department of Energy (DOE), Office of Basic Energy Sciences, Division of Materials Sciences and Engineering (contract no. DE-AC0276SF00515). C.L.B. acknowledges financial support from the Ramsay Memorial Fellowship Trust. Both M.K. and P.R.U. acknowledge support from Warwick-Monash Alliance Accelerator funding. Separately, P.R.U. acknowledges support from a Royal Society Wolfson Research Merit Award. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF)/Stanford Nano-fabrication Facility (SNF), supported by the National Science Foundation under award ECCS-1542152. D.H.A. and N.J.S. acknowledge support from the DOE Office of Basic Energy Sciences SBIR program under awards DE-SC-0007691 and DE-SC-0009573. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. We thank J. Lim, S. B. Kalirai, C. Baeumer, L. Zhang, Y.-L. Liang and C. E. D. Chidsey for discussions and assistance with the experiments.
Author information
Authors and Affiliations
Contributions
J.T.M. and W.C.C. developed the concept of the experiments, J.T.M. performed the synthesis, STXM, UV–vis, EQCM, RDE, SEM and XRD experiments. A.R.A. performed the EC-AFM and TEM experiments. P.R.U., M.K. and C.L.B. designed and performed the SECCM experiments. W.E.G. wrote the principal components analysis and non-negative matrix factorization code for the STXM data analysis. H.D.D. performed the EC-AFM image alignment. D.H.A. and N.J.S. designed and fabricated the STXM cell. Y.-S.Y. and D.A.S. assisted with the STXM experiments. All authors contributed to the discussion of the results and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.H.A. and N.J.S. are employed by Hummingbird Scientific, which designed and manufactured the STXM microfluidic liquid cell used in these experiments.
Additional information
Peer review information Nature thanks Shannon Boettcher, Marcel Risch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains supplementary text, supplementary figures 1 – 12, supplementary equations and supplementary references.
Rights and permissions
About this article
Cite this article
Mefford, J.T., Akbashev, A.R., Kang, M. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021). https://doi.org/10.1038/s41586-021-03454-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03454-x
This article is cited by
-
Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction
Nature Catalysis (2024)
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
High-spin Co3+ in cobalt oxyhydroxide for efficient water oxidation
Nature Communications (2024)
-
Recent progress of cobalt-based electrocatalysts for water splitting: Electron modulation, surface reconstitution, and applications
Nano Research (2024)
-
Semimetallic hydroxide materials for electrochemical water oxidation
Science China Materials (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.