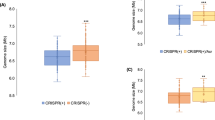
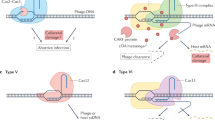
Abstract
Horizontal gene transfer and mutation are the two major drivers of microbial evolution that enable bacteria to adapt to fluctuating environmental stressors1. Clustered, regularly interspaced, short palindromic repeats (CRISPR) systems use RNA-guided nucleases to direct sequence-specific destruction of the genomes of mobile genetic elements that mediate horizontal gene transfer, such as conjugative plasmids2 and bacteriophages3, thus limiting the extent to which bacteria can evolve by this mechanism. A subset of CRISPR systems also exhibit non-specific degradation of DNA4,5; however, whether and how this feature affects the host has not yet been examined. Here we show that the non-specific DNase activity of the staphylococcal type III-A CRISPR–Cas system increases mutations in the host and accelerates the generation of antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis. These mutations require the induction of the SOS response to DNA damage and display a distinct pattern. Our results demonstrate that by differentially affecting both mechanisms that generate genetic diversity, type III-A CRISPR systems can modulate the evolution of the bacterial host.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data for the DNA sequencing of the rpoB gene and the staphylococcal genome after the daily phage infections performed in this study can be found at the NCBI BioProject records PRJNA683580 and PRJNA683848, respectively. Our custom Python script MutPosGraph can be accessed at https://github.com/marraffinilab/Distance-Between-Mutant-Calculator.
References
Polz, M. F., Alm, E. J. & Hanage, W. P. Horizontal gene transfer and the evolution of bacterial and archaeal population structure. Trends Genet. 29, 170–175 (2013).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Chen, J. S. et al. CRISPR–Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018).
Kazlauskiene, M., Tamulaitis, G., Kostiuk, G., Venclovas, Č. & Siksnys, V. Spatiotemporal control of type III-A CRISPR–CAS immunity: coupling DNA degradation with the target RNA recognition. Mol. Cell 62, 295–306 (2016).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Tang, T. H. et al. Identification of novel non-coding RNAs as potential antisense regulators in the archaeon Sulfolobus solfataricus. Mol. Microbiol. 55, 469–481 (2005).
Garneau, J. E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Hale, C. R. et al. RNA-guided RNA cleavage by a CRISPR RNA–Cas protein complex. Cell 139, 945–956 (2009).
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Goldberg, G. W., Jiang, W., Bikard, D. & Marraffini, L. A. Conditional tolerance of temperate phages via transcription-dependent CRISPR–Cas targeting. Nature 514, 633–637 (2014).
Rostøl, J. T. & Marraffini, L. A. Non-specific degradation of transcripts promotes plasmid clearance during type III-A CRISPR–Cas immunity. Nat. Microbiol. 4, 656–662 (2019).
Kazlauskiene, M., Kostiuk, G., Venclovas, Č., Tamulaitis, G. & Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR–Cas systems. Science 357, 605–609 (2017).
Niewoehner, O. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017).
Samai, P. et al. Co-transcriptional DNA and RNA cleavage during type III CRISPR–Cas Immunity. Cell 161, 1164–1174 (2015).
Liu, T. Y., Liu, J. J., Aditham, A. J., Nogales, E. & Doudna, J. A. Target preference of type III-A CRISPR–Cas complexes at the transcription bubble. Nat. Commun. 10, 3001 (2019).
Didier, J. P. et al. Impact of ciprofloxacin exposure on Staphylococcus aureus genomic alterations linked with emergence of rifampin resistance. Antimicrob. Agents Chemother. 55, 1946–1952 (2011).
Rosche, W. A. & Foster, P. L. Determining mutation rates in bacterial populations. Methods 20, 4–17 (2000).
El Meouche, I. & Dunlop, M. J. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science 362, 686–690 (2018).
Jiang, W., Samai, P. & Marraffini, L. A. Degradation of phage transcripts by CRISPR-associated RNases enables type III CRISPR–Cas immunity. Cell 164, 710–721 (2016).
Luria, S. E. & Delbrück, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Gerrish, P. A simple formula for obtaining markedly improved mutation rate estimates. Genetics 180, 1773–1778 (2008).
Maslowska, K. H., Makiela-Dzbenska, K. & Fijalkowska, I. J. The SOS system: a complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 60, 368–384 (2019).
Mo, C. Y. et al. Systematically altering bacterial SOS activity under stress reveals therapeutic strategies for potentiating antibiotics. mSphere 1, e00163-16 (2016).
Slilaty, S. N. & Little, J. W. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc. Natl Acad. Sci. USA 84, 3987–3991 (1987).
Miller, J. H. & Low, K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell 37, 675–682 (1984).
Łobocka, M. et al. Genomics of staphylococcal Twort-like phages—potential therapeutics of the post-antibiotic era. Adv. Virus Res. 83, 143–216 (2012).
Ayora, S. et al. Double-strand break repair in bacteria: a view from Bacillus subtilis. FEMS Microbiol. Rev. 35, 1055–1081 (2011).
Cirz, R. T. et al. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 3, e176 (2005).
Pyenson, N. C., Gayvert, K., Varble, A., Elemento, O. & Marraffini, L. A. Broad targeting specificity during bacterial type III CRISPR–Cas immunity constrains viral escape. Cell Host Microbe 22, 343–353 (2017).
Cao, L. et al. Identification and functional study of type III-A CRISPR–Cas systems in clinical isolates of Staphylococcus aureus. Int. J. Med. Microbiol. 306, 686–696 (2016).
Diep, B. A. et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739 (2006).
Denamur, E. et al. Intermediate mutation frequencies favor evolution of multidrug resistance in Escherichia coli. Genetics 171, 825–827 (2005).
Wang, S., Wang, Y., Shen, J., Wu, Y. & Wu, C. Polymorphic mutation frequencies in clinical isolates of Staphylococcus aureus: the role of weak mutators in the development of fluoroquinolone resistance. FEMS Microbiol. Lett. 341, 13–17 (2013).
Silas, S. et al. Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase–Cas1 fusion protein. Science 351, aad4234 (2016).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR–Cas14 enzymes. Science 362, 839–842 (2018).
Gill, S. R. et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187, 2426–2438 (2005).
Bae, T., Baba, T., Hiramatsu, K. & Schneewind, O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62, 1035–1047 (2006).
Kreiswirth, B. N. et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305, 709–712 (1983).
Horinouchi, S. & Weisblum, B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 150, 815–825 (1982).
Khan, S. A. & Novick, R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid 10, 251–259 (1983).
Horinouchi, S. & Weisblum, B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J. Bacteriol. 150, 804–814 (1982).
Morton, T. M., Johnston, J. L., Patterson, J. & Archer, G. L. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob. Agents Chemother. 39, 1272–1280 (1995).
Andrews, J. M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48 (Suppl 1), 5–16 (2001).
Kearse, M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Wichelhaus, T. A. et al. Biological cost of rifampin resistance from the perspective of Staphylococcus aureus. Antimicrob. Agents Chemother. 46, 3381–3385 (2002).
Bae, T. & Schneewind, O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 (2006).
Cirz, R. T. et al. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J. Bacteriol. 189, 531–539 (2007).
Kennedy, S. et al. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat. Protocols 9, 2586–2606 (2014).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Husmann, L. K., Scott, J. R., Lindahl, G. & Stenberg, L. Expression of the Arp protein, a member of the M protein family, is not sufficient to inhibit phagocytosis of Streptococcus pyogenes. Infect. Immun. 63, 345–348 (1995).
Charpentier, E. et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 70, 6076–6085 (2004).
Bikard, D. et al. Exploiting CRISPR–Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 32, 1146–1150 (2014).
Kinnevey, P. M. et al. Emergence of sequence type 779 methicillin-resistant Staphylococcus aureus harboring a novel pseudo staphylococcal cassette chromosome mec (SCCmec)-SCC-SCCCRISPR composite element in Irish hospitals. Antimicrob. Agents Chemother. 57, 524–531 (2013).
Acknowledgements
We thank D. Bikard for constructing plasmid pDB236, and C. Lai, N. Nnatubeugo and X. Qiu for their advice on and assistance with the Illumina library preparation procedure. C.Y.M. is supported by a National Institutes of Health (NIH) NRSA postdoctoral fellowship (1F32GM128271-01); A.V. is supported by the Arnold O. Beckman Postdoctoral Fellowship; J.T.R. is supported by a Boehringer Ingelheim Fonds PhD fellowship; and D.V.B. is supported by an NIH Medical Scientist Training Program grant (T32GM007739) to the Weill Cornell–Rockefeller–Sloan Kettering Tri-Institutional MD–PhD Program. Support for this work comes from the NIH Director’s Pioneer Award 1DP1GM128184-01 and the Burroughs Wellcome Fund PATH Award to L.A.M. L.A.M. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Experiments were designed by C.Y.M. and L.A.M. C.Y.M. conducted all experiments with help from J.M. A.V. constructed and tested the S. aureus JAV6 strain. J.T.R. constructed and tested plasmid pJTR127. D.V.B. constructed and tested plasmid pDVB51. The paper was written by C.Y.M. and L.A.M.
Corresponding authors
Ethics declarations
Competing interests
L.A.M. is a cofounder and Scientific Advisory Board member of Intellia Therapeutics, and a co-founder of Eligo Biosciences.
Additional information
Peer review information Nature thanks Ivan Matic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Type III-A CRISPR–Cas immunity against the pG0400 conjugative plasmid and the ϕNM1γ6 phage increases the mutation frequency and rate of the staphylococcal host.
a, Schematic of the S. epidermidis RP62a type III-A locus showing the mutants analysed in this study. The CRISPR array shows repeats as black boxes and spacers as coloured, numbered boxes. b, Model of the type III-A CRISPR–Cas immune response. A Cas10 complex, composed of Cas10 and Csm2–Csm5 (in different shades of green), is loaded with a crRNA guide after processing of the transcript of the CRISPR array by Cas6 (not shown). The crRNA guide is used to direct the Cas10 complex to a complementary transcript produced by the invader following infection. Target recognition triggers two activities of Cas10. The Palm domain catalyses the conversion of ATP into a cyclic tetra- or hexa-adenosyl ring that serves as a second messenger that binds and activates Csm6, a non-specific RNase. Degradation of both cellular and invader transcripts by this nuclease results in the growth arrest of the host; this growth arrest is required for the clearance of plasmid and phage targets that have mismatches with the crRNA guide or that are transcribed either weakly or late in the phage lytic cycle. In addition, the HD domain of Cas10 is activated, leading to the non-specific degradation of ssDNA. This activity is believed to be concentrated on the ssDNA generated at the invader’s transcription bubble or within R-loops such as those formed during transcription elongation by the RNA polymerase. Finally, the Csm3 or Cmr4 subunit of the Cas10 complex cleaves the target transcript, turning off both enzymatic activities of Cas10. In this Article, we show that the ssDNase activity of type III-A CRISPR–Cas immunity can also lead to an increase in the frequency and rate of mutation of the host, presumably through degradation of other ssDNA regions of the host chromosome. c, Calculation of P values using a two-sided Mann–Whitney test of the data presented in Fig. 1b for the mutation frequency of wild-type S. epidermidis RP62a, without the two outlier data points in the pG0400 wild-type samples. Horizontal bars, median values; n (pG0400 wild type) = 14 biologically independent experiments; n (pG0400 mutant, none) = 16 biologically independent experiments. d, Staphylococcus aureus cells (around 109) that were treated with ϕNM1γ6 phage and survived infection through the targeting of an early-expressed gene by the staphylococcal type II-A or type III-A CRISPR–Cas systems were seeded after 2 h of outgrowth on plates with or without rifampicin to calculate their mutation frequency. e, Staphylococcus aureus cells (fewer than 1,000) that were treated with ϕNM1γ6 phage and survived infection through the targeting of an early-expressed gene (gp12 or gp14) by the staphylococcal type II-A or type III-A CRISPR–Cas systems were seeded on plates with or without rifampicin to calculate their mutation frequency. f, Calculation of the mutation rate using the data presented in e. g, Staphylococcus aureus cells (fewer than 1,000) that were treated with ϕNM1γ6 phage and survived infection through the targeting of a late-expressed gene (gp43) by type III-A CRISPR–Cas immunity were seeded on plates with or without rifampicin to calculate their mutation frequency. In d, e, g, box limits, interquartile range; whiskers, minimum to maximum; centre line, median; dots, individual data points; n = 15 biologically independent experiments; P values obtained with a two-sided Mann–Whitney test. h, Calculation of the mutation rate using the data presented in g. In f, h, the bar graphs represent the mean; the error bars represent 95% confidence intervals.
Extended Data Fig. 2 Mutagenesis mediated by type III-A CRISPR–Cas immunity against phage infection.
a, Serial tenfold dilutions of ϕNM1γ6 phage were spotted on plates seeded with different strains of staphylococci expressing a gp14- or gp43-targeting S. epidermidis type III-A system with different mutations, wild-type LexA or LexA(Ind-), or the gp12-targeting S. aureus type II-A system. Plaque-forming units (PFU) were enumerated; mean ± s.e.m.; n = 4 (non-targeting control) or n = 6 (type II-A CRISPR immunity) biologically independent experiments. In cases in which plaques were not readily visible, the limit of detection of three biologically independent experiments was reported. b, Staphylococcus aureus cells (fewer than 1,000) that were treated with ϕNM1γ6 phage and survived infection through the targeting of an early-expressed gene (gp14) by different mutant versions of the type III-A CRISPR–Cas system, in the presence of an active (wild-type) or inactive (lexA(Ind-)) SOS response, were seeded on plates with or without rifampicin to calculate their mutation frequency. c, Calculation of the mutation rate using the data presented in b. The bar graphs represent the mean; the error bars represent 95% confidence intervals. d, Staphylococcus aureus cells (around 109) that were treated with ϕNM1γ6 phage and survived infection through the targeting of a late-expressed gene (gp43) by either a wild-type or a dcas10 type III-A CRISPR–Cas system, were seeded on plates with or without rifampicin to calculate their mutation frequency. In b, d, box limits, interquartile range; whiskers, minimum to maximum; centre line, median; dots, individual data points; n = 15 biologically independent experiments; P values obtained with a two-sided Mann–Whitney test.
Extended Data Fig. 3 Type III-A CRISPR–Cas immunity against plasmids with inducible targets.
a, Staphylococcus aureus TB4 strains were transformed with pCRISPR plasmids containing the dcsm3/dcsm6 or the dcas10/dcsm3/dcsm6 mutations in the type III-A CRISPR–Cas locus. Strains were then transformed with a plasmid expressing an inducible transcript with a target (pTarget, top gels) or non-target (pControl, bottom gels) sequence. Cultures were induced with anhydrotetracycline and samples were collected at the indicated time points. Plasmids were extracted, linearized by restriction digestion, separated and visualized with agarose gel electrophoresis. The experiment was performed once. ‘M’, molecular weight marker. b, Calculation of the mutation rate of the data presented in Fig. 3b. The bar graphs represent the mean; the error bars represent 95% confidence intervals. c, Calculation of the mutation rate of wild-type S. epidermidis RP62a and the ΔCRISPR-mutant. The bar graphs represent the mean; the error bars represent 95% confidence intervals; n = 10 biologically independent experiments.
Extended Data Fig. 4 Abrogation of SOS induction in the lexA(Ind-) mutant.
a, A mutation in lexA that prevents its self-cleavage and the induction of the SOS response, K156A, was previously identified in Escherichia coli25. Alignment with the LexA sequence of S. aureus identified K168 as the homologous residue, which was mutated in S. aureus TB4 to alanine to generate strain JAV6. b, Strains TB4 and JAV6 were transformed with an SOS reporter plasmid, pAV22, carrying the GFP ORF downstream of the promoter for the 4-oxalocrotonate tautomerase gene. This promoter is activated after LexA self-cleavage when the SOS response is induced with MMC. Each strain was either treated or not with MMC and both the growth (OD600) and green fluorescence (arbitrary units, A.U.) were measured over time, to report the fluorescence/growth ratio. c, qPCR of the SOS-induced polV transcript after infection (with ϕNM1γ6) of cells that have a wild-type type III-A system in either wild-type or lexA(Ind-) hosts, as well as cells with the dcas10 mutation. RNA was collected when the cultures reached an OD600 of 0.15 (exponential growth phase), after infection at an MOI of 10, and used for qPCR, using the housekeeping gene rho as an internal reference for each sample. The mean of three independent biological replicates ± s.d. is reported. P values obtained with a two-sided t-test.
Extended Data Fig. 5 Distribution of rpoB mutations detected through next-generation sequencing.
DNA from ϕNM1γ6- or rifampicin-resistant staphylococci with different genetic backgrounds was extracted and subjected to next-generation sequencing. Mutations that localized to either of the two clusters that are known to contain amino acid substitutions that confer rifampicin resistance (cluster I: Gly462–Gly489; cluster II: Pro515–Leu530) were counted. The percentage of the total mutations in these clusters is shown for different nucleotides within codons. Different data markers indicate were used to indicate different mutations. Numerical values of data points are provided in Supplementary Data 2.
Extended Data Fig. 6 Mutagenesis mediated by type III-A CRISPR–Cas immunity increases resistance to gentamicin but not to fusidic acid.
a, Staphylococcus aureus cells (fewer than 1,000) that were treated with ϕNM1γ6 phage and survived infection through the targeting of an early-expressed gene (gp14) by the type III-A CRISPR–Cas system, were seeded on plates with or without gentamicin to calculate their mutation frequency. Box limits, interquartile range; whiskers, minimum to maximum; centre line, median; dots, individual data points; n = 15 biologically independent experiments; P values obtained with a two-sided Mann–Whitney test. b, Calculation of the mutation rate using the data presented in in a. The bar graphs represent the mean; the error bars represent 95% confidence intervals.
Extended Data Fig. 7 Type III-A CRISPR–Cas immunity against ϕStaph1N phage.
a, Serial tenfold dilutions of ϕStaph1N phage were spotted on plates seeded with different strains of staphylococci expressing a gp14-targeting S. epidermidis type III-A system with different mutations, wild-type LexA or LexA(Ind-), or the S. aureus type II-A system. b, PCR amplification of the CRISPR arrays of the wild-type pCRISPR plasmid isolated from 16 individual colonies resistant to ϕStaph1N phage that were previously exposed to or not treated with ϕNM1γ6 phage. Molecular markers are shown on the left; arrowheads mark the PCR products of the non-expanded CRISPR array.
Extended Data Fig. 8 Distribution of mutations generated during the type III-A CRISPR–Cas immune response against ϕNM1γ6 phage.
a, CFU per microlitre of wild-type and dcas10/dcsm3 cultures after 6, 12, 18 and 24 daily infections with ϕNM1γ6 phage. Bar graphs represent the mean CFU μl−1; individual black dots represent the individual values from the five independent cultures. b, Distribution of mutations in the S. aureus TB4 genome of wild-type and dcas10/dcsm3 cells infected daily with ϕNM1γ6 phage after 18 days. Genomic DNA from the cultures was labelled with unique molecular barcodes to reduce sequencing errors, subjected to next-generation sequencing and analysed with the appropriate software for this procedure (https://github.com/Kennedy-Lab-UW/Duplex-Sequencing). Mutations were counted if a particular genomic position possessed a minimal depth of 10 reads. The x axis represents the position within the S. aureus TB4 genome; the y axis represents the number of mutations divided by the sequencing depth at that particular position. Replicate 1 shows the data collected from a single wild-type or dcas10/dcsm3 culture; replicate 2 shows data from the pooled five cultures of each strain. On the basis of this analysis, the samples do not show an obvious mutational hotspot in the genome. c, Distance (in base pairs) between adjacent mutations in the S. aureus TB4 genome, calculated from the data obtained in b.
Extended Data Fig. 9 Effects of type III-A CRISPR–Cas immunity on self and non-self DNA.
During type III-A CRISPR–Cas immunity the ssDNase activity of Cas10 (HD domain) is activated by the recognition of a target transcript produced by the invading phage or plasmid. On the one hand, destruction of foreign DNA by this activity provides immunity and restricts evolution through horizontal gene transfer. On the other hand, non-specific ssDNA degradation can also damage the host chromosome, leading to the induction of the SOS response and error-prone repair, and facilitating evolution through mutagenesis.
Supplementary information
Supplementary Figure 1
Raw gel images of (a) Fig. 1c, (b) Extended Data Fig. 2a, (c) Extended Data Fig. 3a, (d) Extended Data Fig. 7a, (e) Extended Data Fig. 7b. Blue rectangle, area cropped for the figure.
Supplementary Tables
This file contains Supplementary Tables 1-3, which list the stains, oligos and plasmids used in the study.
Supplementary Data 1
Numeric values obtained for experiments performed in this study.
Supplementary Data 2
Next-generation sequencing analysis of rpoB mutations.
Rights and permissions
About this article
Cite this article
Mo, C.Y., Mathai, J., Rostøl, J.T. et al. Type III-A CRISPR immunity promotes mutagenesis of staphylococci. Nature 592, 611–615 (2021). https://doi.org/10.1038/s41586-021-03440-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03440-3
This article is cited by
-
RNA-targeting CRISPR–Cas systems
Nature Reviews Microbiology (2023)
-
Alternative functions of CRISPR–Cas systems in the evolutionary arms race
Nature Reviews Microbiology (2022)
-
Structural and biochemical characterization of in vivo assembled Lactococcus lactis CRISPR-Csm complex
Communications Biology (2022)
-
Digging into the lesser-known aspects of CRISPR biology
International Microbiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.