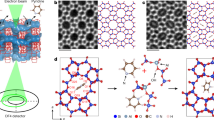
Abstract
Single-molecule imaging is challenging but highly beneficial for investigating intermolecular interactions at the molecular level1,2,3,4,5,6. Van der Waals interactions at the sub-nanometre scale strongly influence various molecular behaviours under confinement conditions7,8,9,10,11. Inspired by the traditional compass12, here we use a para-xylene molecule as a rotating pointer to detect the host–guest van der Waals interactions in the straight channel of the MFI-type zeolite framework. We use integrated differential phase contrast scanning transmission electron microscopy13,14,15 to achieve real-space imaging of a single para-xylene molecule in each channel. A good correlation between the orientation of the single-molecule pointer and the atomic structure of the channel is established by combining the results of calculations and imaging studies. The orientations of para-xylene help us to identify changes in the van der Waals interactions, which are related to the channel geometry in both spatial and temporal dimensions. This work not only provides a visible and sensitive means to investigate host–guest van der Waals interactions in porous materials at the molecular level, but also encourages the further study of other single-molecule behaviours using electron microscopy techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon request.
References
Lee, H. J. & Ho, W. Single-bond formation and characterization with a scanning tunneling microscope. Science 286, 1719–1722 (1999).
Lantz, M. A. et al. Quantitative measurement of short-range chemical bonding forces. Science 291, 2580–2583 (2001).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Albers, B. J. et al. Three-dimensional imaging of short-range chemical forces with picometre resolution. Nat. Nanotechnol. 4, 307–310 (2009).
Gross, L. et al. Organic structure determination using atomic-resolution scanning probe microscopy. Nat. Chem. 2, 821–825 (2010).
Zhang, J. et al. Real-space identification of intermolecular bonding with atomic force microscopy. Science 342, 611–614 (2013).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Lai, Z. et al. Microstructural optimization of a zeolite membrane for organic vapor separation. Science 300, 456–460 (2003).
Rosi, N. L. et al. Hydrogen storage in microporous metal–organic frameworks. Science 300, 1127–1129 (2003).
Li, J. R., Kuppler, R. J. & Zhou, H. C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Bereciartua, P. J. et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017).
Carlson, J. B. Lodestone compass: Chinese or Olmec primacy? Science 189, 753–760 (1975).
Lazić, I., Bosch, E. G. T. & Lazar, S. Phase contrast STEM for thin samples: integrated differential phase contrast. Ultramicroscopy 160, 265–280 (2016).
Lazić, I., Bosch, E. G. T., Lazar, S., Wirix, M. & Yücelen, E. Integrated differential phase contrast (iDPC)—direct phase imaging in STEM for thin samples. Microsc. Microanal. 22, 36–37 (2016).
Lazić, I. & Bosch, E. G. T. in Advances in Imaging and Electron Physics Vol. 199 (ed. Hawkes, P. W.) Ch. 3, 75–184 (Elsevier, 2017).
Margenau, H. Van der Waals forces. Rev. Mod. Phys. 11, 1–35 (1939).
Israelachvili, J. N. Intermolecular and Surface Forces Ch. 6, 107–129 (Academic, 2011).
Aradhya, S. V., Frei, M., Hybertsen, M. S. & Venkataraman, L. Van der Waals interactions at metal/organic interfaces at the single-molecule level. Nat. Mater. 11, 872–876 (2012).
Béguin, L., Vernier, A., Chicireanu, R., Lahaye, T. & Browaeys, A. Direct measurement of the van der Waals interaction between two Rydberg atoms. Phys. Rev. Lett. 110, 263201 (2013).
Kawai, S. et al. Van der Waals interactions and the limits of isolated atom models at interfaces. Nat. Commun. 7, 11559 (2016).
Yücelen, E., Lazić, I. & Bosch, E. G. T. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution. Sci. Rep. 8, 2676 (2018).
Song, D. et al. Visualization of dopant oxygen atoms in a Bi2Sr2CaCu2O8+δ superconductor. Adv. Funct. Mater. 29, 1903843 (2019).
de Graaf, S., Momand, J., Mitterbauer, C., Lazar, S. & Kooi, B. J. Resolving hydrogen atoms at metal-metal hydride interfaces. Sci. Adv. 6, eaay4312 (2020).
Shen, B., Chen, X., Shen, K., Xiong, H. & Wei, F. Imaging the node-linker coordination in the bulk and local structures of metal-organic frameworks. Nat. Commun. 11, 2692 (2020).
Shen, B. et al. Atomic spatial and temporal imaging of local structures and light elements inside zeolite frameworks. Adv. Mater. 32, 1906103 (2020).
Kokotailo, G. T., Lawton, S. L., Olson, D. H. & Meier, W. M. Structure of synthetic zeolite ZSM-5. Nature 272, 437–438 (1978).
van Koningsveld, H., Tuinstra, F., Van Bekkum, H. & Jansen, J. C. The location of p-xylene in a single crystal of zeolite H-ZSM-5 with a new, sorbate-induced, orthorhombic framework symmetry. Acta Crystallogr. B 45, 423–431 (1989).
Goyal, R., Fitch, A. N. & Jobic, H. Powder neutron and X-ray diffraction studies of benzene adsorbed in zeolite ZSM-5. J. Phys. Chem. B 104, 2878–2884 (2000).
Taylor, J. C. Hexadeuteriobenzene locations in the cavities of ZSM-5 zeolite by powder neutron diffraction. J. Chem. Soc. Chem. Commun. 15, 1186–1187 (1987).
Fyfe, C. A., Lee, J. S. J., Cranswick, L. M. D. & Swainson, I. Powder neutron diffraction determination of the structure of the o-xylene/zeolite ZSM-5 complex. Microporous Mesoporous Mater. 112, 299–307 (2008). f
Fyfe, C. A. & Brouwer, D. H. Optimization, standardization, and testing of a new NMR method for the determination of zeolite host-organic guest crystal structures. J. Am. Chem. Soc. 128, 11860–11871 (2006).
Fyfe, C. A., Diaz, A. C., Grondey, H., Lewis, A. R. & Förster, H. Solid state NMR method for the determination of 3D zeolite framework/sorbate structures: 1H/29Si CP MAS NMR study of the high-loaded form of p-xylene in ZSM-5 and determination of the unknown structure of the low-loaded form. J. Am. Chem. Soc. 127, 7543–7558 (2005).
Boronat, M. & Corma, A. What is measured when measuring acidity in zeolites with probe molecules? ACS Catal. 9, 1539–1548 (2019).
LeBeau, J. M., Findlay, S. D., Allen, L. J. & Stemmer, S. Position averaged convergent beam electron diffraction: theory and applications. Ultramicroscopy 110, 118–125 (2010).
Chen, Q. et al. Imaging beam-sensitive materials by electron microscopy. Adv. Mater. 32, 1907619 (2020).
Kirkland, E. J. Advanced Computing in Electron Microscopy 3rd edn (Springer, 2020).
Bosch, E. G. T. & Lazić, I. Analysis of HR-STEM theory for thin specimen. Ultramicroscopy 156, 59–72 (2015).
Bosch, E. G. T. & Lazić, I. Analysis of depth-sectioning STEM for thick samples and 3D imaging. Ultramicroscopy 207, 112831 (2019).
Allen, L. J., D’Alfonso, A. J. & Findlay, S. D. Modelling the inelastic scattering of fast electrons. Ultramicroscopy 151, 11–22 (2015).
Gao, W. et al. Real-space charge-density imaging with sub-ångström resolution by four-dimensional electron microscopy. Nature 575, 480–484 (2019).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 25, 1463–1473 (2004).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Acknowledgements
We thank Q. Zhang and W. Gao for discussions. This work was supported by the National Key Research and Development Program of China (2018YFB0604801), the National Natural Science Foundation of China (21771029, 202013981 and 22005170) and the National Key R&D Program of China (2017YFB0602204). Y.H. thanks the Center Applied Research Fund (FCC/1/1974-16-01) from King Abdullah University of Science and Technology. We thank the Tsinghua National Laboratory for Information Science and Technology for assistance with the energy simulation.
Author information
Authors and Affiliations
Contributions
B.S., X.C. and F.W. conceived this project and designed the studies; B.S. and X.C. performed the electron microscopy experiments and data analysis; H.W. carried out the energy calculations; H.W. and H.X. prepared the zeolite samples; E.G.T.B. and I.L. performed the simulation of iDPC-STEM images; D.C., W.Q., S.J., X.L. and Y.H. helped with the data analysis; and B.S. and X.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Bruce Gates, Xiaodong Zou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Atomic structure of MFI-type framework.
a–c, The structure of the MFI-type framework. d–f, iDPC-STEM images of the empty ZSM-5 framework taken along the three main crystallographic axes.
Extended Data Fig. 2 Shapes of ZSM-5 crystals.
a, ADF-STEM image showing the shapes of pristine short-b-axis ZSM-5 crystals. b, ADF-STEM images showing the hollow shapes of etched ZSM-5 crystals (quasi-2D areas are marked with red boxes).
Extended Data Fig. 3 Measuring sample thickness using the PACBED method.
a, ADF-STEM image of the same ZSM-5 crystal as in Fig. 2. b, Experimental PACBED pattern at the thin area in a (marked by the red spot) and simulated PACBED patterns with different sample thicknesses. c, Analysis of PACBED patterns by comparing the profiles of experimental and simulated patterns. All profiles are along the same direction, marked by the red arrow in b. The experimental data fall between those of the simulated profiles with thicknesses of 4 nm and 6 nm. d, Least-squares fitting of the experimental PACBED patterns with the simulated patterns. The inset shows the magnified profiles in c. The fitting also indicates that the thickness is most likely to be between 4 nm and 6 nm.
Extended Data Fig. 4 Measuring sample thickness using other methods.
a, ADF-STEM image of an etched ZSM-5 crystal from the lateral view ([100] direction). b, Profile analysis of the thin areas from the lateral view. c, iDPC-STEM image of the lateral lattice of an etched ZSM-5 crystal, showing a thickness of about 4 nm. d, ADF-STEM image of etched ZSM-5 crystals with an obvious thin area. e, Thickness mapping and profile analysis obtained by electron energy loss spectroscopy. The measured thickness is about 4 nm. It should be noted that there are uncertainties in these thickness values, mainly due to errors in estimating the mean free path for inelastic scattering. f, ADF-STEM image of an etched ZSM-5 crystal for PX imaging. g, Profile analysis of the etched ZSM-5 crystal in f. This profile includes the image intensities relative to the background along the direction marked by the red arrow in f. h, Relation between ADF-STEM intensity and sample thickness. The intensities are obtained from the intensity profiles of ADF-STEM images, and the thicknesses are measured by the least-squares fitting of PACBED patterns. On the basis of the intensity of the thin area in g (around 0.61 kcounts), we can obtain the thickness of this area as 4–6 nm. i, iDPC-STEM image of the thin area marked by the red box in f. The PX molecules in the channels can be clearly seen.
Extended Data Fig. 5 Simulations of iDPC-STEM imaging.
a, Simulated iDPC-STEM images with different numbers of PX molecules and ZSM-5 unit cells (that is, different ratios of PX and ZSM-5 thickness). The top four images are the simulated results with an ideal probe and no noise. The bottom four images include the noise, the probe sizes and high-pass filtering based on the experimental conditions. b, Profile analysis of the simulated iDPC-STEM images compared with the experimental images. The normalized intensity profiles of three channels (three coloured arrows shown in the top three images) with different ratios of PX to ZSM-5 thickness indicate that the height of PX peak is related to this ratio of PX to ZSM-5. The heights of 10 PX peaks in 10 channels obtained in Fig. 2c are just between those of 1 PX in 2 unit cells and 1 PX in 3 unit cells. Because the measured thickness of the quasi-2D area is 4–6 nm (2–3 ZSM-5 unit cells), the PX number in each channel can only be one.
Extended Data Fig. 7 Correlation between PX orientation and channel geometry.
a, b, Distance ratios of Si1–Si6/Si3–Si8 (a) and Si2–Si7/Si3–Si8 (b) and their relation to the angles between the PX orientation and the a axis of the ZSM-5 crystal. The insets give the mean values and the standard deviations (error bars) of distance ratios when we summarized the data with the same PX orientations together. Both of these ratios show a notable downward trend with increasing orientation angle, which reveals the correlation between PX orientation and channel geometry.
Extended Data Fig. 8 Real-time change of PX orientation.
iDPC-STEM images continuously captured from the same area. The red boxes highlight the same channel in each image—the channel in which we analysed the change of channel geometry and the rotation of the PX molecule in Fig. 4 (note that there is a small shift in the sample between images).
Extended Data Fig. 9 Energy calculations with changing channel geometry.
Time-dependent change of the calculated interaction energy when each PX orientation was fixed and only the channel geometry was changed, as observed in Fig. 4. The green boxes indicate the lowest energy at each time, which is usually the minimum point on the curve of each PX orientation.
Rights and permissions
About this article
Cite this article
Shen, B., Chen, X., Wang, H. et al. A single-molecule van der Waals compass. Nature 592, 541–544 (2021). https://doi.org/10.1038/s41586-021-03429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03429-y
This article is cited by
-
In situ imaging of the atomic phase transition dynamics in metal halide perovskites
Nature Communications (2023)
-
Encapsulating and stabilizing enzymes using hydrogen-bonded organic frameworks
Nature Protocols (2023)
-
Dynamic active-site induced by host-guest interactions boost the Fenton-like reaction for organic wastewater treatment
Nature Communications (2023)
-
Anomalous efficiency elevation of quantum-dot light-emitting diodes induced by operational degradation
Nature Communications (2023)
-
A metal-free photoactive nitrogen-doped carbon nanosolenoid with broad absorption in visible region for efficient photocatalysis
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.