Abstract
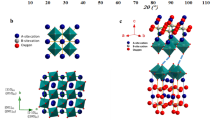
One challenge for the commercial development of solid oxide fuel cells as efficient energy-conversion devices is thermo-mechanical instability. Large internal-strain gradients caused by the mismatch in thermal expansion behaviour between different fuel cell components are the main cause of this instability, which can lead to cell degradation, delamination or fracture1,2,3,4. Here we demonstrate an approach to realizing full thermo-mechanical compatibility between the cathode and other cell components by introducing a thermal-expansion offset. We use reactive sintering to combine a cobalt-based perovskite with high electrochemical activity and large thermal-expansion coefficient with a negative-thermal-expansion material, thus forming a composite electrode with a thermal-expansion behaviour that is well matched to that of the electrolyte. A new interphase is formed because of the limited reaction between the two materials in the composite during the calcination process, which also creates A-site deficiencies in the perovskite. As a result, the composite shows both high activity and excellent stability. The introduction of reactive negative-thermal-expansion components may provide a general strategy for the development of fully compatible and highly active electrodes for solid oxide fuel cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Shao, Z. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Gao, Z., Mogni, L. V., Miller, E. C., Railsback, J. G. & Barnett, S. A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 9, 1602–1644 (2016).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019); author correction 5, 729 (2020).
Zhang, Y. et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 29, 1700132 (2017).
Wei, B. et al. Crystal structure, thermal expansion and electrical conductivity of perovskite oxides BaxSr1−xCo0.8Fe0.2O3−δ (0.3 ≤ x ≤ 0.7). J. Eur. Ceram. Soc. 26, 2827–2832 (2006).
Xia, C., Rauch, W., Chen, F. & Liu, M. Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid State Ion. 149, 11–19 (2002).
Esquirol, A., Brandon, N. P., Kilner, J. A. & Mogensen, M. Electrochemical characterization of La0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 151, A1847–A1855 (2004).
Wang, W. G. & Mogensen, M. High-performance lanthanum-ferrite-based cathode for SOFC. Solid State Ion. 176, 457–462 (2005).
Zhou, W., Ran, R. & Shao, Z. Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3−δ-based cathodes for intermediate-temperature solid-oxide fuel cells: a review. J. Power Sources 192, 231–246 (2009).
Zhou, W., Shao, Z., Ran, R., Jin, W. & Xu, N. A novel efficient oxide electrode for electrocatalytic oxygen reduction at 400–600 °C. Chem. Commun. 44, 5791–5793 (2008).
Chen, D. et al. Evaluation of pulsed laser deposited SrNb0.1Co0.9O3-δ thin films as promising cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 295, 117–124 (2015).
Zhou, W., Ran, R., Shao, Z., Jin, W. & Xu, N. Evaluation of A-site cation-deficient (Ba0.5Sr0.5)1−xCo0.8Fe0.2O3−δ (x>0) perovskite as a solid-oxide fuel cell cathode. J. Power Sources 182, 24–31 (2008).
Liu, J., Co, A. C., Paulson, S. & Birss, V. I. Oxygen reduction at sol–gel derived La0.8Sr0.2Co0.8Fe0.2O3 cathodes. Solid State Ion. 177, 377–387 (2006).
Chen, Y., Shen, J., Yang, G., Zhou, W. & Shao, Z. A single-/double-perovskite composite with an overwhelming single-perovskite phase for the oxygen reduction reaction at intermediate temperatures. J. Mater. Chem. A 5, 24842–24849 (2017).
Baek, S. W., Kim, J. H. & Bae, J. Characteristics of ABO3 and A2BO4 (A=Sm, Sr; B=Co, Fe, Ni) samarium oxide system as cathode materials for intermediate temperature-operating solid oxide fuel cell. Solid State Ion. 179, 1570–1574 (2008).
Wang, F., Zhou, Q., He, T., Li, G. & Ding, H. Novel SrCo1−yNbyO3−δ cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 195, 3772–3778 (2010).
Hrovat, M., Holc, J. & Kolar, D. Thick film ruthenium oxide/yttria-stabilized zirconia-based cathode material for solid oxide fuel cells. Solid State Ion. 68, 99–103 (1994).
Zhou, Q., Wang, F., Shen, Y. & He, T. Performances of LnBaCo2O5+x–Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 195, 2174–2181 (2010).
Zhou, W., Shao, Z., Ran, R. & Cai, R. Novel SrSc0.2Co0.8O3-δ as a cathode material for low temperature solid-oxide fuel cell. Electrochem. Commun. 10, 1647–1651 (2008).
Ding, X., Cui, C. & Guo, L. Thermal expansion and electrochemical performance of La0.7Sr0.3CuO3−δ–Sm0.2Ce0.8O2−δ composite cathode for IT-SOFCs. J. Alloys Compd. 481, 845–850 (2009).
Song, Y. et al. A cobalt-free multi-phase nanocomposite as near-ideal cathode of intermediate-temperature solid oxide fuel cells developed by smart self-assembly. Adv. Mater. 32, 1906979 (2020).
Mary, T. A., Evans, J. S., Vogt, T. & Sleight, A. W. Negative thermal expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 272, 90–92 (1996).
Chen, J. et al. Zero thermal expansion in PbTiO3-based perovskites. J. Am. Chem. Soc. 130, 1144–1145 (2008).
Goodwin, A. L. & Kepert, C. J. Negative thermal expansion and low-frequency modes in cyanide-bridged framework materials. Phys. Rev. B 71, 140301 (2005).
Forster, P. M. & Sleight, A. W. Negative thermal expansion in Y2W3O12. Int. J. Inorg. Mater. 1, 123–127 (1999).
Sumithra, S., Waghmare, U. V. & Umarji, A. M. Anomalous dynamical charges, phonons, and the origin of negative thermal expansion in Y2W3O12. Phys. Rev. B 76, 024307 (2007).
Khaliullin, S. M., Khaliullina, A. S. & Neiman, A. Y. High-temperature conductivity and structure of Y2(WO4)3 ceramics. Russ. J. Phys. Chem. B 10, 62–68 (2016).
Zhou, W., Jin, W., Zhu, Z. & Shao, Z. Structural, electrical and electrochemical characterizations of SrNb0.1Co0.9O3−δ as a cathode of solid oxide fuel cells operating below 600 °C. Int. J. Hydrogen Energy 35, 1356–1366 (2010).
Rosen, B. A. Progress and opportunities for exsolution in electrochemistry. Electrochem 1, 32–43 (2020).
Han, H. et al. Lattice strain-enhanced exsolution of nanoparticles in thin films. Nat. Commun. 10, 1471 (2019); author correction 10, 2083 (2019).
Zhu, Y. et al. An A-site-deficient perovskite offers high activity and stability for low-temperature solid-oxide fuel cells. ChemSusChem 6, 2249–2254 (2013).
Zhu, Y. et al. Influence of crystal structure on the electrochemical performance of A-site-deficient Sr1-sNb0.1Co0.9O3-δ perovskite cathodes. RSC Advances 4, 40865–40872 (2014).
Duan, C., Hook, D., Chen, Y., Tong, J. & O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 10, 176–182 (2017).
Biesinger, M. C. et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011).
Lu, Q., Chen, Y., Bluhm, H. & Yildiz, B. Electronic structure evolution of SrCoOx during electrochemically driven phase transition probed by in situ X-ray spectroscopy. J. Phys. Chem. C 120, 24148–24157 (2016).
Li, M., Zhou, W., Peterson, V. K., Zhao, M. & Zhu, Z. A comparative study of SrCo0.8Nb0.2O3−δ and SrCo0.8Ta0.2O3−δ as low-temperature solid oxide fuel cell cathodes: effect of non-geometry factors on the oxygen reduction reaction. J. Mater. Chem. A 3, 24064–24070 (2015).
Koo, B. et al. Sr segregation in perovskite oxides: why it happens and how it exists. Joule 2, 1476–1499 (2018).
Lee, K. T. & Manthiram, A. Comparison of Ln0.6Sr0.4CoO3−δ (Ln=La, Pr, Nd, Sm, and Gd) as cathode materials for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 153, A794–A798 (2006).
Das, S., Das, S. & Das, K. Synthesis and thermal behavior of Cu/Y2W3O12 composite. Ceram. Int. G. 40, 6465–6472 (2014).
Ganesh, V. V. & Gupta, M. Effect of the extent of reinforcement interconnectivity on the properties of an aluminum alloy. Scr. Mater. 44, 305–310 (2001).
Uju, W. A. & Oguocha, I. N. A. A study of thermal expansion of Al–Mg alloy composites containing fly ash. Mater. Des. 33, 503–509 (2012).
Barbucci, A. et al. Influence of electrode thickness on the performance of composite electrodes for SOFC. J. Appl. Electrochem. 38, 939–945 (2008).
Liu, L., Kim, G. Y. & Chandra, A. Modeling of thermal stresses and lifetime prediction of planar solid oxide fuel cell under thermal cycling conditions. J. Power Sources 195, 2310–2318 (2010).
Zhang, Y. et al. Significantly improving the durability of single-chamber solid oxide fuel cells: a highly active CO2 -resistant perovskite cathode. ACS Appl. Energy Mater. 1, 1337–1343 (2018).
Wan, T. H., Saccoccio, M., Chen, C. & Ciucci, F. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRT tools. Electrochim. Acta 184, 483–499 (2015).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21576135, 21878158, 21828801 and 52006150), Jiangsu Natural Science Foundation for Distinguished Young Scholars (BK20170043), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and State Key Laboratory of Materials-Oriented Chemical Engineering. R.O. acknowledges support from the Fulbright Foundation Global Scholars Program and the US Army Research Office under grant number W911NF-17-540 1-0051. M.N. acknowledges a Research Grant Council University Grants Committee Hong Kong SAR Grant, reference number PolyU 152064/18E. The authors also acknowledge the assistance on HRTEM observation received from the Electron Microscope Center of Shenzhen University.
Author information
Authors and Affiliations
Contributions
W.Z., R.O. and Z.S. conceived and designed the project. Y.Z. and B.C. performed the characterizations and experiments. Y.Z., B.C. and D.G. analysed the data. M.X., R.R. and M.N. contributed the laboratory apparatus and experiment sites. Y.Z., B.C., W.Z., R.O. and Z.S. drafted the article and revised it critically. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Yanhai Du, Anke Hagen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains the source images for Figs 1 and 3.
Supplementary Information
This file contains Supplementary Sections 1–4, including Supplementary Tables 1–8, Supplementary Figures 1–19 and Supplementary References.
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, B., Guan, D. et al. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 591, 246–251 (2021). https://doi.org/10.1038/s41586-021-03264-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03264-1
This article is cited by
-
Experimental study on current distribution in parallel-connected solid oxide fuel cell strings
Frontiers in Energy (2024)
-
Recent progresses in the development of tubular segmented-in-series solid oxide fuel cells: Experimental and numerical study
International Journal of Minerals, Metallurgy and Materials (2024)
-
Simple chemical synthesis and isotropic negative thermal expansion in MHfF6 (M = Ca, Mn, Fe, and Co)
Nano Research (2024)
-
Anomalous thermal expansion and enhanced magnetocaloric effect in <001>-textured MnxFe5–xSi3 alloys
Rare Metals (2024)
-
Sintering-induced cation displacement in protonic ceramics and way for its suppression
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.