Abstract
With rapidly changing ecology, urbanization, climate change, increased travel and fragile public health systems, epidemics will become more frequent, more complex and harder to prevent and contain. Here we argue that our concept of epidemics must evolve from crisis response during discrete outbreaks to an integrated cycle of preparation, response and recovery. This is an opportunity to combine knowledge and skills from all over the world—especially at-risk and affected communities. Many disciplines need to be integrated, including not only epidemiology but also social sciences, research and development, diplomacy, logistics and crisis management. This requires a new approach to training tomorrow’s leaders in epidemic prevention and response.
Similar content being viewed by others
Main
When Nature published its first issue in 18691, a new understanding of infectious diseases was taking shape. The work of William Farr2, Ignaz Semmelweis3, Louis-René Villermé4 and others had been published; John Snow had traced the source of a cholera epidemic in London5 (although Robert Koch had not yet isolated the bacterium that caused it6). The science of epidemiology has described patterns of disease in human populations, investigated the causes of those diseases, evaluated attempts to control them7 and has been the foundation for public health responses to epidemic infections for over 100 years. Despite great technological progress and expansion of the field, the theories and practices of infectious disease epidemiology are struggling to keep pace with the transitional nature of epidemics in the twenty-first century and the breadth of skills needed to respond to them.
Epidemiological transition theory has focused mostly on the effects of demographic and socioeconomic transitions on well-known preventable infections and a shift from infectious diseases to non-communicable diseases8. However, it has become clear that current demographic transitions—driven by population growth, rapid urbanization, deforestation, globalization of travel and trade, climate change and political instability—also have fundamental effects on the dynamics of infectious diseases that are more difficult to predict. The vulnerability of populations to outbreaks of zoonotic diseases such as Ebola, Middle East respiratory syndrome (MERS) and Nipah has increased, the rise and spread of drug-resistant infections, marked shifts in the ecology of known vectors (for example, the expanding range of Aedes mosquitoes) and massive amplification of transmission through globally connected, high-density urban areas (particularly relevant to Ebola, dengue, influenza and severe acute respiratory syndrome-related coronavirus SARS-CoV). These factors and effects combine and interact, fuelling more-complex epidemics.
Although rare compared to those diseases that cause the majority of the burden on population health, the nature of such epidemics disrupts health systems, amplifies mistrust among communities and creates high and long-lasting socioeconomic effects, especially in low- and middle-income countries. Their increasing frequency demands attention. As the Executive Director of the Health Emergencies Program at the World Health Organization (WHO) has said: “We are entering a very new phase of high-impact epidemics… This is a new normal, I don’t expect the frequency of these events to reduce.”9.
We have to act now but act differently: a broader foundation is required, enhancing traditional epidemiology and public health responses with knowledge and skills from a number of areas (Table 1). Many of these areas have long been associated with epidemic preparedness and response, but they must now stop being seen as esoteric ‘nice things to have’, and instead become fully integrated into the critical planning and response to epidemics.
This will require considerable changes by the global public health community in the way that we respond to epidemics today and how we prepare for and seek to prevent those of tomorrow. It will mean reshaping the global health architecture of the response to epidemics and transforming how we train new generations of researchers and practitioners for the epidemics of the future10.
The modern research culture—often shaped by the behaviour of funders—has required many researchers to specialize in narrow fields, with less emphasis on translation than on field-specific innovations. Although this siloed landscape has brought major advances in global health, it is not fit for the transitional phase of epidemic diseases: rapidly evolving, high-impact events bring together communities, responders and researchers who do not routinely interact. Different assumptions, cultures and practices, each of which may be widely accepted within a particular community, make working together in outbreak situations more challenging. Fundamental to success is respect and understanding of the contribution each party brings. In a successfully integrated approach, we each have to realize that our knowledge and skills are a small part of a rapidly expanding toolkit (Box 1). We need to understand major trends in research and how and when they may influence the response to an epidemic, develop new research to strengthen the support that we can provide across other areas and learn to operate in multi-stakeholder situations—including, at times, as part of a critical debate to bring better practices to the fore.
Central to this approach must be the communities who are at risk and those affected by epidemics: local people are the first responders to any outbreak and their involvement in the preparation and response activities is essential. From communities, through local and regional health authorities, national public health institutes and international organizations—including many essential partners in sectors beyond public health—the integrated approach must be supported. The WHO, in particular, has a critical part to play, using its unique mandate not to lead every aspect of preparation, response and recovery, but to change its practices, facilitate integration with and among others, and ensure accountabilities are built in from the bottom to the top.
Nineteenth and twentieth century epidemiology
A wave of cholera epidemics across Europe in the 1830s and 1840s catalysed a new era of ‘infectious disease diplomacy’11 globally. Nations recognized that infections do not stop at borders and that therefore multilateral collaboration is essential to protecting citizens from lethal epidemics. The development of germ theory through the second half of the nineteenth century12 transformed ideas about the causes of infections, informing scientific research as well as clinical responses. Scientific understanding translated into vaccines13 and antibiotics, while programmes for child health, hygiene, clean water and sanitation became common in the twentieth century. As a result, childhood diseases such as measles and mumps became rare, smallpox was eventually eradicated14 and polio was eliminated from all but a handful of countries15. Many people thought that infectious diseases would soon be history. Sir Frank Macfarlane Burnet is often cited for his remark in the 1970s that, with the emergence of new diseases being a distant prospect, “the future of infectious diseases will be very dull”16.
Although the focus in high-income nations turned to non-communicable diseases, which constituted a considerable and increasing burden on the health of their citizens, infectious diseases did not disappear. Some endemic infections such as malaria and tuberculosis were not susceptible to elimination strategies, and new diseases with epidemic and pandemic potential emerged. Ebola virus disease was first identified in the 1970s, HIV/AIDS in the 1980s, Nipah virus in the 1990s, SARS and MERS at the start of the twenty-first century, and many more have since been identified. Far from becoming ‘very dull’, the field of infectious disease epidemiology has sometimes struggled to adapt: as late as 1990, respected researchers used a nineteenth century ‘law’ of epidemiology to make predictions about the AIDS epidemic—these turned out to be vast underestimates17. Advances in other fields gave epidemiology the chance to evolve. In 2001, when the editors of the International Journal of Epidemiology provocatively asked whether it was time to ‘call it a day’18 given the putative power of genomics to explain diseases over the capacity of epidemiologists to describe them, their conclusion was that it had the potential to positively transform epidemiology as much as the rise of germ theory a century earlier.
The new normal
At least 150 pathogens that affect humans have been identified as emerging, re-emerging or evolving since the 1980s19, while increasing rates of antimicrobial resistance threaten to make formerly controlled infections, such as malaria, untreatable20—this also limits our ability to control their epidemic potential. The demographic transition is driving much of this: human society is becoming more urban than rural for the first time in our history, bringing large numbers of people (and often animals) together in densely populated areas21. Agricultural and forestry practices are changing the relationships between people, animals and our respective habitats22. Travel is more accessible around the world, so migration, trade and tourism bring more people into contact and thus affect disease transmission23. Climate change has many effects on ecosystems and environments, not least in changing the habitats and migratory habits of disease vectors24. States with weak health systems are far less likely to cope with or recover from multiple emergent demands without damaging routine services25. Inequalities26, inequities and distrust in national structures and institutions compound people’s vulnerabilities27. Conflict increases the risk of epidemics and makes responding to them close to impossible28.
Since 2000, there have been several outbreaks of Ebola (including the two biggest in history), not to mention outbreaks of SARS, MERS, Nipah, influenza A subtype H5N1, yellow fever, Zika and the continued spread of dengue. Epidemics overlap and run into each other, yet the world is not currently equipped to cope with this increasing burden of multiple public health emergencies. Preparing for epidemics, therefore, requires global health, economic and political systems to be integrated just as much as infectious disease epidemiology, translational research and development, and community engagement.
Essential areas in epidemic response
Governance and infrastructure
Epidemics represent shared risks that cross borders and all of society. Health systems, routine care, trust in governments, travel, trade, business—all are disrupted during an epidemic. With such broad risks, the preparation and response must be nationally owned and led, internationally supported and undertaken with a whole-of-society approach. Some initiatives have started to build frameworks for this to happen in a coordinated way. For example, the WHO’s Pandemic Influenza Preparedness Framework brings together nation states, industry, other stakeholders and the WHO to implement a global approach to pandemic preparedness and response29.
A focus must be building coordinated regional and country expertise, resources and capacity through national and regional public health institutions30. This brings its own challenges—governance of institutions, leadership, collaborations and interventions have to be impeccable or misconduct can thrive31. Unwelcome in itself, misuse of funding, resources or people within efforts intended to support an epidemic response will also undermine trust in the organizations that respond to an outbreak and, in turn, prolong the outbreak.
Key governance components include drafting policies in advance and being willing to implement those policies for data collection and sharing during epidemics. They must be flexible enough to enable affected communities and nations to retain ownership of the response, while drawing on international expertise to find the best possible response. Governance should also include processes for vaccine and therapeutic approvals during outbreaks. However, it is clear that the centre of gravity for leadership, governance and implementation must be where the need is greatest if these are to truly deliver.
In 1971, Julian Tudor Hart proposed the inverse care law: “The availability of good medical care tends to vary inversely with the need for it in the population served.”32. An analogue of the inverse care law can be applied to public health and epidemiology. Expertise in these fields has traditionally gravitated towards centres of excellence in Europe and the United States. Of course, high-income countries are not immune to the disruption associated with epidemics, especially in an era of misinformation and growing mistrust in authorities and public health initiatives. However, the centre of gravity must shift so that globally representative distributed networks of collaborating centres can jointly ensure coverage in the regions that urgently need these skills on the ground33. International collaborations remain important; however, strengthening epidemiology, public health and laboratory capacity in low- and middle-income countries is essential34. Collaborative interventions should not be limited to when there is a major outbreak, but be integrated into regular interactions.
Capacity, resources, expertise and governance can be supported by the increasing role for regional and national centres of disease control. The US Centers for Disease Control (CDC) lends its expertise all around the world in addition to protecting the US population. In 2004, the European CDC started, followed by the China CDC in 2015 and by the Africa CDC in 2017. Although more can be done to improve data sharing and access to laboratories, the networks and connections between these centres have strengthened all of their work, as well as having a positive effect on public health systems in low- and middle-income countries.
Engagement and communication
During the pan-European wave of cholera in the 1830s, there were riots across the continent: doctors, nurses and pharmacists were murdered, hospitals and medical equipment destroyed27. Similar reports today usually come from communities that have not had positive prior interactions with public health initiatives, and thus the encounter with national or international teams who arrive only in response to a ‘new’ disease means that trust can never be assumed and has to be earned on both sides. Engagement needs to start before an outbreak—ensuring that patients, their families and their communities are at the centre of all public health is essential for the successful prevention and response to epidemics. There is no public health without the support of the community.
For example, the early detection of disease events will be improved if more national and regional public health institutions establish community event-based surveillance systems. Communities are the first to know when something unusual happens35—therefore training and mobilizing community volunteers to report such occurrences is a cost-effective way to rapidly detect diseases and contain them at the source. This will also help to sustain engagement between communities and the organizations that respond to outbreaks. Furthermore, improved information flow between the community and the public health system should provide a better understanding of local social networks to complement other means of tracking chains of transmission between individuals and places. This can be the community themselves, or it might be veterinarians who see clusters of sick animals, or nurses and doctors who care for patients in primary care—or it may be teams that are often forgotten in public health initiatives, such as those working in critical care facilities; it is striking how the first cases of Nipah, SARS, MERS and influenza A subtype H5N1 were all first identified by clinical teams in critical care facilities.
An inclusive, whole-of-society approach is challenging, and the challenges may be magnified in a conflict or post-conflict zone. Wars and conflicts not only increase the risk of epidemics as people move to escape violence and health services become harder to maintain36, but also make public health responses vulnerable to interruption, thus making them less effective. Then, miscommunication, mistrust, disease and violence can fuel each other in a vicious cycle. Engaging local communities remains the highest priority, even in unstable contexts such as North Kivu and Ituri provinces of the Democratic Republic of the Congo (DRC)37, where an Ebola epidemic started in August 2018. It seems inevitable that responding to epidemics in politically unstable environments will become more common, and skilled negotiators and peacekeepers will have to be better integrated in response teams. Equally essential, therefore, will be an improved understanding of these challenging operational contexts among affected communities and external responders alike.
Social sciences
Social scientists have long applied their skills and knowledge in epidemic responses, although their roles have become more visible in recent years38. By focusing on communities, social science humanizes the epidemic response39, helps to increase understanding of context and may uncover associations between the context or local practices and the risk of transmission. The Social Science in Humanitarian Action Platform40 has successfully produced rapid reports and briefings on regions in which an epidemic has been identified, and the Global Research Collaboration for Infectious Disease Preparedness includes a social science research funders’ forum to ‘propel research in this area’41, acknowledging that its integration in the preparation and response to outbreaks is often missing or added as an afterthought to solve a problem that could have been forseen. There is still much to learn about how epidemic responders and social scientists can make the most of each other’s expertise42 and how data from social science can fit into the wider information architecture of epidemic response.
As an example, behavioural surveillance43 will be critical in twenty-first century responses to disease outbreaks44. Just as behavioural surveillance to improve the understanding of HIV was crucial in identifying high-risk groups for HIV infection, so human behaviours will continue to be important as we respond to future infectious diseases. For instance, the Ebola virus outbreak in West Africa probably began before December 2013, but it took several months before hospital transmission and traditional burial practices were found to be the leading causes of its rapid spread.
Emerging technologies
The increasing prevalence of mobile phones, wireless internet connectivity and social media activity raises the possibility of using these tools to gather data for epidemiological studies, diagnostics45, population mobility during an Ebola epidemic46 or influenza incidence in real time47. Future developments in predictive technology, machine learning and artificial intelligence will bring more opportunities to move towards ‘precision public health’ (Box 2).
The use of data from people is becoming strictly controlled, however, and it will be a challenge to persuade countries to invest in a new surveillance system, for example, before its general effectiveness has been demonstrated at a country level48. Even then, technology-based solutions should be integrated with community-based programmes and other existing epidemic preparedness and response systems because surveillance is more effective when standardized among different countries, districts and communities. To this end, suites of guidance and open-access standardized tools are being developed for reporting cases of disease, as well as consent forms, standard operating procedures and training materials49, properly validated diagnostic assays and access to quality-assurance panels in public50 and veterinary51 health. The rising trend of engaging citizens in data gathering is also welcome—the use of mosquito-recognition apps enables the collection of data far beyond the capacity of routine mosquito surveillance52. This way, citizens feed information into the public health system and the feedback loop offers a fast and direct way to provide citizens with details of potential actions that they can take.
As well as potentially supporting diagnosis and surveillance53, the fast-developing field of genomic epidemiology54 can yield information to track the evolution of a virus such as Ebola during an epidemic55,56. There will be times when it can detect outbreaks better than traditional epidemiology, illustrating the need to have these tools available in the same toolbox. During the large Lassa fever outbreak in Nigeria in 2018, real-time genomic sequencing provided clear evidence that the rapid increase was not due to a single Lassa virus variant, nor attributable to sustained human-to-human transmission. Rather, the outbreak was characterized by vast viral diversity defined by geography, with major rivers acting as barriers to migration of the rodent reservoir57. These findings were crucial in containing the outbreak.
Developing and sustaining the capacity to conduct real-time sequencing with adequate bioinformatics analyses at regional and national levels will be challenging in low- and middle-income countries. Moreover, investments in relatively high-tech capacity (such as real-time sequencing) are competing with other, arguably more fundamental needs, such as equipment and training in primary laboratories. Political engagement must be nurtured between epidemics: it is not enough to offer technological and laboratory support during a crisis, even with the promise of building capacity, if the political will is not there. However, with proper preparation, and accessible and trusted data sharing and governance mechanisms, laboratories with limited resources may be able to leap-frog into the twenty-first century58,59.
Research and development
Vaccination is one of the most effective public health interventions and innovative strategies for research and development of vaccines, such as using ring vaccination as a trial design during Ebola epidemics since 201560,61,62, must be encouraged. At the start of the 2013–2015 epidemic in West Africa, vaccine candidates were already in development, based on a long history of preclinical research, although a lot of work was still required to get clinical trials underway in time to be useful63. In 2015, when Zika was first internationally recognized as a pathogen that could cause birth defects64, there was hardly any research and no vaccines in late-stage development. Two-and-a-half years later, results from three phase I clinical trials had been reported65, although challenges remained for further development. The lack of a profitable market for such products means that pharmaceutical companies lack the incentives to push this work between epidemics. Initiatives such as the Coalition for Epidemic Preparedness Innovations are attempting to positively disrupt financing models for vaccines against epidemic diseases66, and stockpiles of meningococcal vaccine, yellow fever vaccine and oral cholera vaccine are maintained by the International Coordinating Group to minimize potential delays due to limited manufacturing capacity67.
Similarly, if investigational treatments or vaccines are to be used as part of the response to an epidemic, ethical protocols68 for managing informed consent and introducing them in clinical settings must be planned in advance with at-risk communities (Box 3). Trial designs69 should be created as soon as the option becomes viable. The essential consideration is how the resulting data can add to previous trials and influence the approach to trials in future epidemics. For example, research during the 2013–2015 Ebola epidemic enabled progress on therapeutic agents70 that are now being trialled in the ongoing outbreak in DRC71. Scientific progress during and between epidemics must be matched by other workstreams, such as the preparation of supply chain logistics and communication with at-risk populations. Plans have to be made for a series of future outbreaks, enabling adaptive, multi-year, multi-country studies72. Similar plans are needed for continual preclinical research to ensure that future vaccine and therapeutic pipelines will be filled.
One Health
The term ‘One Health’73 is used to acknowledge that human, animal and ecosystem health are tightly interconnected and need to be studied in the context of each other (Fig. 1). Changes in the environment—whether natural or anthropogenic—affect interactions between pathogens, vectors and hosts in multiple and complex ways, making the emergence or decline of endemic, epidemic and zoonotic diseases difficult to predict, while epidemics of animal diseases can challenge a community’s access to food. The fact that pools of viruses, bacteria and parasites are maintained in wild and domesticated animals74 makes surveillance of potentially zoonotic diseases an intrinsic part of One Health epidemic planning. Many agencies and nations around the world now use prioritization tools such as those developed by the US CDC75 or the United Nations (UN) Food and Agriculture Organization (FAO)76 to identify and prioritize zoonotic diseases of concern. An early precedent was a joint consultation on emerging zoonotic diseases by the WHO, the FAO and the World Organisation for Animal Health in 200477. Understanding disease ecology in the zoonotic reservoir could potentially lead to ways to predict the risk of human disease, thus providing the basis for smart early-warning surveillance systems.
Individual countries with limited resources for epidemiological studies and epidemic preparation and response must decide their own priorities. However, infectious diseases do not respect borders. Similarly, the interdisciplinary nature of One Health means there are several different lenses through which different sectors assess risks and priorities. For One Health approaches to work, these multiple perspectives must be taken into account, whether human health or animal health, ecology or social sciences78.
Recovery
Epidemics do more than cause death and debilitation: they increase pressure on healthcare systems and healthcare workers and draw resources from services not directly linked to the epidemic. This can leave a legacy of distrust between people, governments and health systems, although more-positive outcomes have been found to strengthen relations between communities and public authorities. The full social and economic costs of the Ebola outbreak in West Africa have been estimated79 to be as high as US$53 billion when including the effect on health workers, long-term conditions suffered by 17,000 Ebola survivors, and costs of treatment, infection control, screening and deployment of personnel beyond West Africa. As healthcare resources became increasingly allocated to the Ebola response, hospital admissions fell and deaths from other diseases rose markedly, adding US$18.8 billion to the estimated cost. Such pressure can be withstood in high-income countries with strong health systems, but in low-income countries the pressure can quickly reach a breaking point.
Ebola killed almost 1.5% of doctors, nurses and midwives in Guinea, 6.85% in Sierra Leone and just over 8% in Liberia80. This is compared to mortality between 0.02% and 0.11% of the whole population of these countries. Estimates of the effect of this loss on maternal mortality suggest that thousands more women may have died in childbirth each year since the epidemic ended. Beyond the tragic deaths of so many healthcare workers, people were less likely to use health services for children or adults during the epidemic, suggesting decreased trust or even fear of healthcare settings81. More recently, in some areas affected by the 2018 Ebola outbreak in DRC, the introduction of free non-Ebola healthcare led to unprecedented demand. However, healthcare facilities were not given sufficient additional resources to care for the number of people, which may have contributed to nosocomial infections.
Survivors, too, need to be cared for long after the epidemic is declared over. A cohort of more than 3,000 children is growing up in Brazil after being born with microcephaly because their mothers were infected with Zika during pregnancy. Tracking the development of these children increases understanding of the effects of Zika infection and helps to define what medical and social support the affected families may need as many of the children will grow up with severe developmental delays82.
Outlook
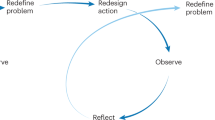
The challenges posed by twenty-first century epidemics are real and changing: future epidemics will be fuelled by conflict, poverty, climate change, urbanization and the broader demographic transition. In our response we must consider epidemics not as discrete events, but rather as connected cycles for which we can prepare, even if we cannot predict specific outbreaks. The challenge is then to choose the right response at the right scale in the right area at the right time. There needs to be a greater emphasis on absorbing and using positive lessons from each episode and avoiding those that led to negative outcomes83.
The way that we train practitioners and researchers working in all fields relevant to today’s epidemic landscape has to change. A modern approach that is capable of characterizing epidemics and the best ways to control them must go beyond a narrow definition of epidemiology that sustains artificial barriers between disciplines. Instead, it must be able to integrate tools and practices from a diverse range of established and emerging scientific, humanistic, political, diplomatic and security fields. We believe that such an approach needs to become the norm for the curriculums of schools of public health around the world.
As well as training new generations of epidemiologists so that they have the skills, knowledge and networks to recognize and make use of every tool available to help them to do their work effectively, the entire architecture of the response to epidemics has to be adapted. Only then will we be able to maintain the comprehensive and effective response—including prevention and research—needed to stop epidemics and protect people’s lives, no matter what the circumstances.
References
Huxley, T. H. Nature: aphorisms by Goethe. Nature 1, 9–11 (1869).
Lilienfeld, D. E. Celebration: William Farr (1807–1883)—an appreciation on the 200th anniversary of his birth. Int. J. Epidemiol. 36, 985–987 (2007).
Kadar, N. Rediscovering Ignaz Philipp Semmelweis (1818–1865). Am. J. Obstet. Gynecol. 220, 26–39 (2019).
Julia, C. & Valleron, A.-J. Louis-Rene Villerme (1782–1863), a pioneer in social epidemiology: re-analysis of his data on comparative mortality in Paris in the early 19th century. J. Epidemiol. Community Health 65, 666–670 (2011).
Fine, P. et al. John Snow’s legacy: epidemiology without borders. Lancet 381, 1302–1311 (2013). This is a wide-ranging meeting report that places modern epidemiology in the context of the past two hundred years and highlights the importance of bringing in new disciplines, remaining open-minded and using those skills across a wider range of societal issues than are traditionally considered public health.
Howard-Jones, N. Robert Koch and the cholera vibrio: a centenary. Br. Med. J. (Clin. Res. Ed.) 288, 379–381 (1984).
Coggon, D., Rose, G. & Barker, D. J. P. Epidemiology for the Uninitiated (BMJ Books, 2003).
Omran, A. R. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem. Fund Q. 49, 509–538 (1971).
Gallagher, J. Large Ebola outbreaks new normal, says WHO. BBC News (7 June 2019).
Brownson, R. C., Samet, J. M. & Bensyl, D. M. Applied epidemiology and public health: are we training the future generations appropriately? Ann. Epidemiol. 27, 77–82 (2017).
WHO. Managing Epidemics: Key Facts about Major Deadly Diseases https://apps.who.int/iris/handle/10665/272442 (WHO, 2018).
Carter, K. C. Ignaz Semmelweis, Carl Mayrhofer, and the rise of germ theory. Med. Hist. 29, 33–53 (1985).
Plotkin, S. History of vaccination. Proc. Natl Acad. Sci. USA 111, 12283–12287 (2014).
Strassburg, M. A. The global eradication of smallpox. Am. J. Infect. Control 10, 53–59 (1982).
Nathanson, N. & Kew, O. M. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am. J. Epidemiol. 172, 1213–1229 (2010).
Macfarlane Burnet, F. & White, D. O. Natural History of Infectious Disease p263 (Cambridge Univ. Press, 1972).
Bregman, D. J. & Langmuir, A. D. Farr’s law applied to AIDS projections. J. Am. Med. Assoc. 263, 1522–1525 (1990).
Smith, G. D. & Ebrahim, S. Epidemiology—is it time to call it a day? Int. J. Epidemiol. 30, 1–11 (2001).
Smith, K. F. et al. Global rise in human infectious disease outbreaks. J. R. Soc. Interface 11, 20140950 (2014).
MacIntyre, C. R. & Bui, C. M. Pandemics, public health emergencies and antimicrobial resistance — putting the threat in an epidemiologic and risk analysis context. Arch. Public Health 75, 54 (2017).
Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 5, 27060 (2015).
Morse, S. S. in Microbial Evolution and Co-Adaptation: A Tribute to the Life and Scientific Legacies of Joshua Lederberg (National Academies Press, 2009).
Vignier, N. & Bouchaud, O. Travel, migration and emerging infectious diseases. EJIFCC 29, 175–179 (2018).
Paaijmans, K. P., Read, A. F. & Thomas, M. B. Understanding the link between malaria risk and climate. Proc. Natl Acad. Sci. USA 106, 13844–13849 (2009).
Boozary, A. S., Farmer, P. E. & Jha, A. K. The Ebola outbreak, fragile health systems, and quality as a cure. J. Am. Med. Assoc. 312, 1859–1860 (2014).
Quinn, S. C. & Kumar, S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur. Bioterror. 12, 263–273 (2014).
Cohn, S. & Kutalek, R. Historical parallels, Ebola virus disease and cholera: understanding community distrust and social violence with epidemics. PLoS Curr. 8, https://doi.org/10.1371/currents.outbreaks.aa1f2b60e8d43939b43fbd93e1a63a94 (2016).
Sharara, S. L. & Kanj, S. S. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. 10, e1004438 (2014).
WHO. Pandemic Influenza Preparedness Framework for the Sharing of Influenza Viruses and Access to Vaccines and Other Benefits (WHO, 2011).
Nkengasong, J. N. How Africa can quell the next disease outbreaks. Nature 567, 147 (2019).The ability to prevent, detect and respond to any health issues will always depend on the local capacity and although international partners can bring complementary expertise and resources, it is the local capacity that is critical; in this article, the authors argue for national investment in public health, health systems, science and local leadership, examples of which are the establishment of the African CDC, the renewed strength of the WHO African regional office and the African Academy of Sciences.
Cheng, M. AP Exclusive: UN health chief orders probe into misconduct. AP News (17 January 2019).
Tudor Hart, J. The inverse care law. Lancet 297, 405–412 (1971).
Kay, S. Africa’s leadership in biomedical research: shifting the center of gravity. Sci. Transl. Med. 7, 314ed13 (2015).Agenda setting, research questions and funding for biomedical research has historically been led from Northern Hemisphere countries in an unequal Northern–Southern Hemisphere relationship; in this article, a determined approach to shift that centre of gravity is outlined, such that the agenda is firmly based where the need is greatest.
Chataway, J. et al. Science granting councils in sub-Saharan Africa: trends and tensions. Sci. Public Policy 46, 620–631 (2019).
International Federation of Red Cross and Red Crescent Societies. Community-Based Surveillance: Guiding Principles (IFRC, 2017).
Gayer, M., Legros, D., Formenty, P. & Connolly, M. A. Conflict and emerging infectious diseases. Emerg. Infect. Dis. 13, 1625–1631 (2007).
Vinck, P., Pham, P. N., Bindu, K. K., Bedford, J. & Nilles, E. J. Institutional trust and misinformation in the response to the 2018–19 Ebola outbreak in North Kivu, DR Congo: a population-based survey. Lancet Infect. Dis. 19, 529–536 (2019).
Bedford, J. et al. Application of social science in the response to Ebola, Équateur Province, Democratic Republic of the Congo. Wkly. Epidemiol. Rec. 94, 19–23 (2019).
Bardosh, K. et al. Towards People-Centred Epidemic Preparedness and Response: From Knowledge to Action (Wellcome Trust, 2019).
UNICEF & IDS. The Social Science in Humanitarian Action Platform https://www.socialscienceinaction.org/ (2019).
GloPID-R. Social Science Research. https://www.glopid-r.org/our-work/social-science-research/ (2018).
Stellmach, D., Beshar, I., Bedford, J., du Cros, P. & Stringer, B. Anthropology in public health emergencies: what is anthropology good for? BMJ Glob. Health 3, e000534 (2018).
Manhart, L. E. & Khosropour, C. M. Launching a new era for behavioural surveillance. Sex. Transm. Infect. 91, 152–153 (2015).
Miller, M. & Hagan, E. Integrated biological–behavioural surveillance in pandemic-threat warning systems. Bull. World Health Organ. 95, 62–68 (2017).
Wood, C. S. et al. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature 566, 467–474 (2019).
Peak, C. M. et al. Population mobility reductions associated with travel restrictions during the Ebola epidemic in Sierra Leone: use of mobile phone data. Int. J. Epidemiol. 47, 1562–1570 (2018).
Broniatowski, D. A., Paul, M. J. & Dredze, M. National and local influenza surveillance through Twitter: an analysis of the 2012–2013 influenza epidemic. PLoS ONE 8, e83672 (2013).
Velasco, E., Agheneza, T., Denecke, K., Kirchner, G. & Eckmanns, T. Social media and internet-based data in global systems for public health surveillance: a systematic review. Milbank Q. 92, 7–33 (2014).
ISARIC. Protocols & Data Tools https://isaric.tghn.org/protocols/ (accessed 18 September 2019).
WHO. Laboratory Network. https://www.who.int/immunization/monitoring_surveillance/burden/laboratory/en/ (2018).
FAO. Enhancing Early Warning Capabilities and Capacities for Food Safety (FAO, 2015).
European Citizen Science Association. Global Mosquito Alert https://ecsa.citizen-science.net/global-mosquito-alert (accessed 12 October 2019).
Quick, J. et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 530, 228–232 (2016).
Tang, P., Croxen, M. A., Hasan, M. R., Hsiao, W. W. & Hoang, L. M. Infection control in the new age of genomic epidemiology. Am. J. Infect. Control 45, 170–179 (2017).
Gire, S. K. et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 345, 1369–1372 (2014).
Tong, Y.-G. et al. Genetic diversity and evolutionary dynamics of Ebola virus in Sierra Leone. Nature 524, 93–96 (2015).
Siddle, K. J. et al. Genomic analysis of Lassa virus during an increase in cases in Nigeria in 2018. N. Engl. J. Med. 379, 1745–1753 (2018).
Aarestrup, F.M. et al. Integrating genome-based informatics to modernize global disease monitoring, information sharing, and response. Emerg. Infect. Dis. 18, e1 (2012).The integration of genomics and other types of data into the surveillance, prevention and response of epidemics is critical and can help to transform the ability to enhance public health; although the tools are now available, it will be key to ensure that these new approaches are fully integrated and not seen as esoteric ivory tower research, but instead as an essential component of twenty-first century epidemiology, public health and epidemics—the next generation of leaders need to be trained in and comfortable across a range of new disciplines.
GMI Global Microbial Identifier https://www.globalmicrobialidentifier.org/ (2018).
Henao-Restrepo, A. M. et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!).Lancet 389, 505–518 (2017).A seminal study that shows that ring vaccination could be used in the midst of a devastating Ebola epidemic and, furthermore, that innovation research can be conducted in an epidemic, trial designs can be adapted without compromising scientific integrity and that Ebola can be prevented through vaccination.
Rid, A. & Miller, F. G. Ethical rationale for the Ebola “ring vaccination” trial design. Am. J. Public Health 106, 432–435 (2016).
Wells, C. R. et al. Ebola vaccination in the Democratic Republic of the Congo. Proc. Natl Acad. Sci. USA 116, 10178–10183 (2019).
Rojek, A., Horby, P. & Dunning, J. Insights from clinical research completed during the West Africa Ebola virus disease epidemic. Lancet Infect. Dis. 17, e280–e292 (2017).
Siedner, M. J., Ryan, E. T. & Bogoch, I. I. Gone or forgotten? The rise and fall of Zika virus. Lancet Public Health 3, e109–e110 (2018).
Barrett, A. D. T. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines 3, 24 (2018).
Burki, T. CEPI: preparing for the worst. Lancet Infect. Dis. 17, 265–266 (2017).
Yen, C. et al. The development of global vaccine stockpiles. Lancet Infect. Dis. 15, 340–347 (2015).
Calain, P. The Ebola clinical trials: a precedent for research ethics in disasters. J. Med. Ethics 44, 3–8 (2018).It is an ethical imperative to consider and implement research in an epidemic setting as, for many epidemic diseases, it is the only time at which to conduct the research that will inform and improve the lives of the individuals affected during epidemic and to ensure that future generations are better prepared; however, such research is challenging at many levels and it is critical to have an ethical framework that guides the research, places individuals and communites at the heart of the research and facilitates the maximum benefit for the maximum number of people, in an equitable way, that is independent of the ability to pay—such a framework is outlined in this paper and is put into the context of social justice and equity.
Lipsitch, M. & Eyal, N. Improving vaccine trials in infectious disease emergencies. Science 357, 153–156 (2017).
Mendoza, E. J., Qiu, X. & Kobinger, G. P. Progression of Ebola therapeutics during the 2014–2015 outbreak. Trends Mol. Med. 22, 164–173 (2016).
Fallah, M. P. & Skrip, L. A. Ebola therapies: an unconventionally calculated risk. Lancet 393, 850–852 (2019).
Brueckner, M., Titman, A., Jaki, T., Rojek, A. & Horby, P. Performance of different clinical trial designs to evaluate treatments during an epidemic. PLoS ONE 13, e0203387 (2018).
Destoumieux-Garzón, D. et al. The One Health concept: 10 years old and a long road ahead. Front. Vet. Sci. 5, 14 (2018).
Day, M. J. et al. Surveillance of zoonotic infectious disease transmitted by small companion animals. Emerg. Infect. Dis. 18, https://doi.org/10.3201/eid1812.120664 (2012).
Rist, C. L., Arriola, C. S. & Rubin, C. Prioritizing zoonoses: a proposed one health tool for collaborative decision-making. PLoS ONE 9, e109986 (2014).
FAO. Evaluation of the Emergency Prevention System (EMPRES) Programme in Food Chain Crises (FAO, 2018).
FAO, WHO & OIE. Report of the WHO/FAO/OIE joint consultation on emerging zoonotic diseases (WHO, 2004).
European Centre for Disease Prevention and Control. Towards One Health preparedness (ECDC, 2018).
Huber, C., Finelli, L. & Stevens, W. The economic and social burden of the 2014 Ebola outbreak in West Africa. J. Infect. Dis. 218, S698–S704 (2018).Epidemics cause enormous disruption to countries, regions and the world; however, the focus is often on the epidemic itself, the pathogen and its immediate effect rather than the much broader effect that the epidemic has not only on the healthcare system—which lasts long after the epidemic itself—as routine vaccination programmes often collapse, maternal–child health suffers, and malaria, HIV and tuberculosis clinics and surgery—all aspects of healthcare—are disrupted, but also on the wider society, as mistrust and tension occurs between citizens, authorities and governments, and education, investments, businesses, trade and tourism inevitablely suffer leading to an economic impact that can be long lasting and devastating for often already fragile communities.
Evans, D. K., Goldstein, M. & Popova, A. Health-care worker mortality and the legacy of the Ebola epidemic. Lancet Glob. Health 3, e439–e440 (2015).
Morse, B., Grépin, K. A., Blair, R. A. & Tsai, L. Patterns of demand for non-Ebola health services during and after the Ebola outbreak: panel survey evidence from Monrovia, Liberia. BMJ Glob. Health 1, e000007 (2016).
Brickley, E. B. & Rodrigues, L. C. Further pieces of evidence in the Zika virus and microcephaly puzzle. Lancet Child Adolesc. Health 2, 162–164 (2018).
Ebola Gbalo Research Group. Responding to the Ebola virus disease outbreak in DR Congo: when will we learn from Sierra Leone? Lancet 393, 2647–2650 (2019).
Miller, D. D. & Brown, E. W. Artificial intelligence in medical practice: the question to the answer? Am. J. Med. 131, 129–133 (2018).
Benke, K. & Benke, G. Artificial intelligence and big data in public health. Int. J. Environ. Res. Public Health 15, 2796 (2018).
McLellan, J. S. et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340, 1113–1117 (2013).
Adalja, A. A., Watson, M., Cicero, A. & Inglesby, T. Vaccine Platforms: State of the Field and Looming Challenges (Johns Hopkins Center for Health Security, 2019).
Charlton Hume, H. K. & Lua, L. H. L. Platform technologies for modern vaccine manufacturing. Vaccine 35, 4480–4485 (2017).
Dowell, S. F., Blazes, D. & Desmond-Hellmann, S. Four steps to precision public health. Nature 540, 189–191 (2016).
Chowkwanyun, M., Bayer, R. & Galea, S. “Precision” public health — between novelty and hype. N. Engl. J. Med. 379, 1398–1400 (2018).
Horton, R. Offline: in defence of precision public health. Lancet 392, 1504 (2018).
Chang, H. H. et al. Mapping imported malaria in Bangladesh using parasite genetic and human mobility data. eLife 8, e43481 (2019).
Krubiner, C. B. et al. Pregnant women & vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine https://doi.org/10.1016/j.vaccine.2019.01.011 (2019).
Heyrana, K., Byers, H. M. & Stratton, P. Increasing the participation of pregnant women in clinical trials. J. Am. Med. Assoc. 320, 2077–2078 (2018).
The Ethics Working Group on ZIKV Research & Pregnancy. Pregnant Women & the Zika Virus Vaccine Research Agenda: Ethics Guidance on Priorities, Inclusion, and Evidence Generation (PREVENT, 2017).
Acknowledgements
We thank M. Regnier at Wellcome for editing the manuscript.
Reviewer information
Nature thanks Peter Byass, Sharon Peacock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors developed the scope and focus of the Review and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bedford, J., Farrar, J., Ihekweazu, C. et al. A new twenty-first century science for effective epidemic response. Nature 575, 130–136 (2019). https://doi.org/10.1038/s41586-019-1717-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1717-y
This article is cited by
-
Six dilemmas for stakeholders inherently affecting data sharing during a zoonotic (re-)emerging infectious disease outbreak response
BMC Infectious Diseases (2024)
-
Emerging and re-emerging pediatric viral diseases: a continuing global challenge
Pediatric Research (2024)
-
Self-stigma in schizophrenia: a systematic review and meta-analysis of 37 studies from 25 high- and low-to-middle income countries
Molecular Psychiatry (2023)
-
COVID-19 pandemic and tourism: The impact of health risk perception and intolerance of uncertainty on travel intentions
Current Psychology (2023)
-
Digital health: trends, opportunities and challenges in medical devices, pharma and bio-technology
CSI Transactions on ICT (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.