Abstract
Nucleic acid-sensing Toll-like receptors (TLRs) are subject to complex regulation to facilitate the recognition of microbial DNA and RNA while limiting the recognition of an organism’s own nucleic acids1. Failure to properly regulate these TLRs can lead to autoimmune and autoinflammatory diseases2,3,4,5,6. Intracellular localization of these receptors is thought to be crucial for the discrimination between self and non-self7, but the molecular mechanisms that reinforce compartmentalized activation of intracellular TLRs remain poorly understood. Here we describe a mechanism that prevents the activation of TLR9 from locations other than endosomes. This control is achieved through the regulated release of the receptor from its trafficking chaperone UNC93B1, which occurs only within endosomes and is required for ligand binding and signal transduction. Preventing release of TLR9 from UNC93B1, either by mutations in UNC93B1 that increase affinity for TLR9 or through an artificial tether that impairs release, results in defective signalling. Whereas TLR9 and TLR3 are released from UNC93B1, TLR7 does not dissociate from UNC93B1 in endosomes and is regulated by distinct mechanisms. This work defines a checkpoint that reinforces the compartmentalized activation of TLR9, and provides a mechanism by which activation of individual endosomal TLRs may be distinctly regulated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are either included within the manuscript or are available from the corresponding author on reasonable request. Source Data for Fig. 4 and Extended Data Fig. 10 are included in the online version of the paper. Gel source data can be found in Supplementary Fig. 1.
References
Majer, O., Liu, B. & Barton, G. M. Nucleic acid-sensing TLRs: trafficking and regulation. Curr. Opin. Immunol. 44, 26–33 (2017).
Deane, J. A. et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Fukui, R. et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity 35, 69–81 (2011).
Mouchess, M. L. et al. Transmembrane mutations in Toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity 35, 721–732 (2011).
Pisitkun, P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Barton, G. M., Kagan, J. C. & Medzhitov, R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 7, 49–56 (2006).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 (2008).
Fukui, R. et al. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J. Exp. Med. 206, 1339–1350 (2009).
Lee, B. L. et al. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife 2, e00291 (2013).
Latz, E. et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 8, 772–779 (2007).
Ohto, U. et al. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 520, 702–705 (2015).
Onji, M. et al. An essential role for the N-terminal fragment of Toll-like receptor 9 in DNA sensing. Nat. Commun. 4, 1949 (2013).
Majer, O., Liu, B., Kreuk, L. S. M., Krogan, N. & Barton, G. M. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature 10.1038/ s41586-019-1612-6 (2019).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Nickerson, K. M. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848 (2010).
Chan, M. P. et al. DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat. Commun. 6, 5853 (2015).
Ewald, S. E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 (2008).
Park, B. et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9, 1407–1414 (2008).
Ewald, S. E. et al. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J. Exp. Med. 208, 643–651 (2011).
Roberts, A. W. et al. Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 47, 913–927.e916, (2017).
Shibata, T. et al. Guanosine and its modified derivatives are endogenous ligands for TLR7. Int. Immunol. 28, 211–222 (2016).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Zhang, Z. et al. Structural analysis reveals that Toll-like receptor 7 is a dual receptor for guanosine and single-stranded RNA. Immunity 45, 737–748 (2016).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Acknowledgements
We thank members of the Barton and Vance laboratory for discussions and critical reading of the manuscript. We thank B. Li and S. Brohawn for supplying the DDM/CHS detergent and for technical advice, A. Yiu-fai Lee and the Gene Targeting Facility of the Cancer Research Laboratory at UC Berkeley for generating the Unc93b1S282A/− knock-in mice, H. Nolla and A. Valeros for assistance with cell sorting at the Flow Cytometry Facility of the Cancer Research Laboratory at UC Berkeley, S. Ruzin and D. Schichnes for assistance with microscopy on the Zeiss Elyra PS.1 at the Biological Imaging Center at UC Berkeley. This work was supported by the National Institutes of Health (NIH; AI072429, AI105184 and AI063302 to G.M.B.) and by the Lupus Research Institute (Distinguished Innovator Award to G.M.B.). O.M. was supported by an Erwin Schrödinger (J 3415-B22) and CRI Irvington postdoctoral fellowship. B.L. was supported by the UC Berkeley Tang Distinguished Scholars Program. B.J.W. was supported by a summer undergraduate research fellowship from UC Berkeley. Research reported in this publication was supported in part by the National Institutes of Health S10 program under award number 1S10OD018136-01.
Author information
Authors and Affiliations
Contributions
O.M. and G.M.B designed experiments. O.M., B.L., B.J.W., L.S.M.K. and E.V.D. performed experiments and analysed the data. O.M. and B.L. performed the initial alanine mutagenesis screen. O.M. wrote the manuscript. G.M.B., O.M. and B.L. revised and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
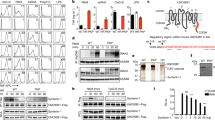
Extended Data Fig. 1 A luminal UNC93B1 mutation results in defective TLR9 signalling despite normal trafficking.
a, Colocalization of UNC93B1 (red) and LAMP1 (green) in macrophages expressing the indicated UNC93B1 alleles using super-resolution structured illumination microscopy. Representative images are shown. Boxed areas are magnified. The plot shows quantification of the percentage of total UNC93B1 within LAMP1+ endosomes. Each dot represents an individual cell. P values determined by unpaired two-tailed Student’s t-test. Data are from a single experiment. Scale bars, 10 µm. b, UNC93B1(S282A) is sufficient for the TLR9 signalling defect. NF-κB luciferase assay in HEK293T cells stimulated with CpG-B (1 µM) for 16 h. Data are normalized to UNC93B1-independent human IL-1β responses and expressed as luciferase fold change over unstimulated controls. n = 3 biological replicates. ***P < 0.0001, determined by unpaired two-tailed Student’s t-test. Blot below shows UNC93B1 expression levels. c, Intracellular cytokine staining of TNF in macrophage lines expressing the indicated UNC93B1 alleles after stimulation with CpG-B (1 µM), R848 (100 ng ml−1), poly(I:C) (100 ng ml−1), Sa19 (a TLR13-specific RNA agonist; 200 ng ml−1), flagellin (100 ng ml−1) or LPS (10 ng ml−1). Grey histograms show unstimulated controls. d, e, TNF production of the indicated macrophage lines after 8 h stimulation with increasing concentrations of CpG-A (d), or LPS (50 ng ml−1) (e). n = 2 biological replicates. ***P < 0.0001, two-way ANOVA followed by a Tukey’s post-test (d) or one-way ANOVA followed by a Tukey’s post-test (e). f, qRT–PCR analysis of Tnfa expression in the indicated macrophage lines 8 h after stimulation with DOTAP–CpG-A (1 µM) or LPS (10 ng ml−1). n = 3 biological replicates. ***P = 0.0003 (S282A versus WT), ***P = 0.0002 (HR versus WT), unpaired two-tailed Student’s t-test. Data are mean ± s.d. and representative of two independent repeats unless noted otherwise.
Extended Data Fig. 2 UNC93B1(S282A) does not affect DNA delivery to TLR9-containing endosomes.
a, Uptake of Cy3-labelled CpG-B (1 µM) of macrophage lines expressing the indicated alleles of UNC93B1–Flag. Data are mean ± s.d. relative uptake compared to wild-type at 60 min. n = 3 biological replicates. P values determined by two-way ANOVA followed by a Tukey’s post-test (WT versus SKN). b, Colocalization of CpG-A (red), TLR9–HA (green) and LAMP1 (magenta) in macrophage lines shown in a after incubation with Cy3-labelled CpG-A (1 µM) for 2 h using super-resolution structured illumination microscopy. Representative images are shown. Boxed areas and areas containing white lines are magnified. The histograms display fluorescent intensity plots of pixels along the white lines. Shaded areas highlight regions of colocalization of CpG-B, TLR9 and LAMP1. c, Quantification of the percentage of CpG-A colocalized with TLR9. Each dot represents an individual cell, n = 4 (WT), n = 5 (SKN) and n = 5 (HR). Data are mean ± s.d. ***P < 0.0001, unpaired two-tailed Student’s t-test. Scale bars, 5 µm. Data are from a single experiment.
Extended Data Fig. 3 UNC93B1(S282A) does not affect TLR9 dimerization or the association between N-terminal and C-terminal cleavage products of TLR9.
a, Macrophage lines co-expressing TLR9–HA and TLR9–V5 together with the indicated UNC93B1–Flag alleles were subjected to haemagglutinin immunoprecipitation followed by V5 immunoblot. TLR9 levels in whole-cell lysates are also shown. b, Macrophage lines expressing TLR9–HA and the indicated UNC93B1–Flag alleles were subjected to immunoprecipitation with an anti-TLR9 antibody specific to the N-terminal cleavage fragment (B33A4), followed by immunoblot of the C-terminal TLR9 fragment with an anti-HA antibody. Data are representative of at least two independent experiments.
Extended Data Fig. 4 UNC93B1(S282A) shows a stronger interaction with TLR9, but not TLR7.
a, The UNC93B1 mutants SKN and S282A display a stronger association with TLR9. Immunoprecipitation of TLR9–HA from macrophage lines expressing the indicated UNC93B1 alleles, followed by immunoblot of UNC93B1–Flag. b, The UNC93B1 mutants SKN and S282A do not affect the interaction with TLR7. Immunoprecipitation of UNC93B1–Flag from macrophage lines expressing TLR7–HA and the indicated UNC93B1 alleles, followed by immunoblot of TLR7–HA. c, Immunoprecipitation of UNC93B1–Flag from macrophage lines expressing the indicated UNC93B1 alleles, followed by immunoblot of TLR9–HA. All blots are representative of at least two independent experiments. KA, K283A; NA, N284A; SA, S282A.
Extended Data Fig. 5 Identification of residues within loop 5 of UNC93B1 that mediate interaction with TLR9.
a, Schematic of the tested loop 5 mutants of UNC93B1 and the relative TLR9 responses indicated in shades of grey; white indicates a response equivalent to wild type, and black indicates no response. Asterisks show human UNC93B1 SNPs that have been tested in f. b, A larger region in loop 5 of UNC93B1 mediates binding to TLR9. Immunoprecipitation of UNC93B1–Flag from macrophage lines expressing the indicated UNC93B1 mutants (spanning amino acids 267–284, and non-functional HR) followed by immunoblot of TLR9–HA. Data are representative of two independent experiments. c, Intracellular cytokine staining of TNF in macrophage lines shown in b after stimulation with CpG-B (25 nM), R848 (100 ng ml−1) or LPS (10 ng ml−1). Shaded histograms show unstimulated controls. Data are representative of three independent experiments. d, Schematics showing relative positions and sequence alignment (bottom) of swapped regions within the TLR9/3 chimaeras. Coloured regions indicate TLR3 sequences. NF-κB luciferase assay in HEK293T cells transiently transfected with the indicated TLR9 and UNC93B1 mutants and stimulated with CpG-B (200 nM) for 16 h. Data are normalized to UNC93B1-independent human IL-1β responses and expressed as luciferase fold change over unstimulated controls. Data are mean ± s.d., n = 3 biological replicates. P values determined by two-way ANOVA followed by a Sidak’s post-test comparing each TLR9 allele co-expressed with UNC93B1 WT versus S282A (TLR9WT: P < 0.0001, TLR9Mut1: P > 0.9999, TLR9Mut2: P < 0.0001, TLR9Mut3: P < 0.0001, TLR9Mut4: P = 0.0020, TLR9Mut5: P = 0.0171). Data are representative experiment of two independent repeats. e, TLR9 mutants that rescue signalling in the presence of UNC93B1(S282A) also show normal binding to UNC93B1(S282A). Haemagglutinin immunoprecipitation of the indicated TLR9 mutants transiently expressed in HEK293T cells stably expressing the indicated UNC93B1–Flag alleles, followed by immunoblot of UNC93B1–Flag. Data are representative of three independent experiments. f, Human UNC93B1 variants with SNPs in loop 5 show decreased TLR9 signalling. NF-κB luciferase assay in HEK293T cells expressing TLR9 or TLR7 and the indicated human UNC93B1–Flag variants and stimulated with CpG-B (250 nM) or R848 (250 ng ml−1) for 16 h, respectively. Data are normalized to Renilla expression and expressed as RLUs. Data are mean ± s.d., n = 3 biological replicates. P values are determined by one-way ANOVA followed by a Tukey’s post-test. For CpG-B stimulations: P = 0.0048 (WT versus G270S), P = 0.0113 (WT versus R277Q), P = 0.9994 (WT versus G283R). Data are representative of four independent experiments. For R848 stimulations: P = 0.2001 (WT versus G270S), P = 0.0002 (WT versus R277Q), P = 0.9933 (WT versus G283R). Data are representative of three independent repeats experiment. g, Expression levels of the UNC93B1 mutants used in f. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 6 Cellular fractionation showing the distribution profiles for CpG-B–biotin ligand and β-hexosaminidase.
Macrophages were stimulated for 4 h with biotinylated CpG-B (1 µM) and subjected to subcellular fractionation by density-gradient centrifugation. The distributions of TLR9–HA, CpG-B, LAMP1 and β-hexosaminidase activity are shown. Data are representative of two independent experiments.
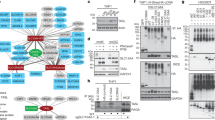
Extended Data Fig. 7 The TLR9–UNC93B1 association is reduced in endosomes compared to the ER.
a, Input controls of TLR9 and UNC93B1 (relates to Fig. 3b). TLR9 and UNC93B1 levels in pooled ER or endosome fractions from macrophage lines expressing TLR9–HA and the indicated UNC93B1 alleles. Data are representative of three independent experiments. b, Increased interaction between TLR9 and UNC93B1(S282A) in endosomes. Immunoprecipitation of UNC93B1–Flag from pooled endosome fractions followed by immunoblot for TLR9–HA. Input controls are also shown. Data are representative of three independent experiments. Bar graph shows the quantification of TLR9 bound to UNC93B1 in pooled endosome fractions, normalized by UNC93B1–Flag levels in endosome fractions. Data are mean ± s.d., each dot represents data from an independent experiment (n = 3). *P = 0.0413, unpaired two-tailed Student’s t-test. c, Release model of TLR9.
Extended Data Fig. 8 Tethering of TLR9 and UNC93B1.
a, Cysteine mutants of TLR9 and UNC93B1 do not affect trafficking of TLR9 to endosomes. Immunoblot of TLR9–HA from macrophage lines expressing the indicated TLR9–HA and UNC93B1–Flag cysteine mutants. Data are representative of two independent experiments. b, TLR9–HA immunoblot under non-reducing conditions after immunoprecipitation of TLR9–HA from macrophage lines shown in a. The high molecular mass band indicates disulfide bond formation between UNC93B1 and TLR9. Data are representative of two independent experiments. c, UNC93B1-tethered TLR9 is unable to signal. NF-κB luciferase assay in HEK293T cells expressing the indicated cysteine mutant combinations and stimulated with CpG-B (1 µM) for 16 h. Data are normalized to Renilla expression and expressed as luciferase fold change over unstimulated controls. Data are mean ± s.d., n = 3 biological replicates. P values determined by unpaired two-tailed Student’s t-test. Data are representative of three independent experiments.
Extended Data Fig. 9 TLR3 but not TLR7 releases from UNC93B1 in endosomes.
a, Subcellular fractionation of macrophages lines showing the distributions of TLR3, TLR7 and TLR9 across fractions. The pooled endosome and ER fractions for subsequent co-immunoprecipitations are highlighted. b, Immunoprecipitation of TLR9–HA and TLR3–HA from pooled ER or endosome fractions of macrophage lines expressing wild-type UNC93B1. Immunoprecipitated TLR–HA levels were normalized across fractions and probed for levels of UNC93B1–Flag. Bar graph shows the quantification of UNC93B1 bound to TLR3 between ER and endosome fractions. Data are mean ± s.d., each dot represents data from an independent experiment (n = 3). **P = 0.0039, paired two-tailed Student’s t-test. c, Input controls of TLR9, TLR7 and UNC93B1 in pooled ER and endosome fractions (relates to Fig. 3d). d, Less TLR3 or TLR9 is associated with wild-type UNC93B1 in pooled endosome fractions compared to TLR7. Immunoprecipitation of UNC93B1–Flag from pooled endosome fractions (as shown in a) followed by immunoblot for TLR3–HA, TLR7–HA or TLR9–HA. Bar graph shows the calculated relative proportion of UNC93B1-bound TLR compared to total amount of the same TLR in the pooled endosome fractions. Data are mean ± s.d., each dot represents data from an independent experiment (n = 3). **P = 0.0032 (TLR3 versus TLR7) and P = 0.0036 (TLR7 versus TLR9), determined by unpaired two-tailed Student’s t-test. All immunoblots are representative of three independent experiments.
Extended Data Fig. 10 Generation of UNC93B1(S282A) knock-in mice and B-cell stimulation with LPS.
a, CRISPR–Cas-9 strategy to generate UNC93B1(S282A) knock-in mice. Blue line indicates the guide sequence. Red bases indicated the edited codon. A representative sequencing trace of genomic DNA from an edited founder mouse is shown. b, B cell proliferation in CSFE-labelled splenocyte cultures of the indicated mouse genotypes after stimulation for 3 days with increasing doses of LPS. The proliferation index is defined as the gMFI of CSFEunstim/CFSEsample, as in Fig. 4c. Each curve shows the dose response of cells from three mice. Data are mean ± s.d. P values determined by two-way ANOVA followed by a Sidak’s post-test. c, Gating strategy for B cell stimulation assay.
Supplementary information
Supplementary Figure 1
This file contains the uncropped gel images for main figures and extended data figures. Separate gels were run for detection of each protein. When loading or normalization controls were used, these samples were run on separate gels.
Rights and permissions
About this article
Cite this article
Majer, O., Liu, B., Woo, B.J. et al. Release from UNC93B1 reinforces the compartmentalized activation of select TLRs. Nature 575, 371–374 (2019). https://doi.org/10.1038/s41586-019-1611-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1611-7
This article is cited by
-
Cyclical palmitoylation regulates TLR9 signalling and systemic autoimmunity in mice
Nature Communications (2024)
-
Regulation of the nucleic acid-sensing Toll-like receptors
Nature Reviews Immunology (2022)
-
Toll-like receptor 9 agonists and combination therapies: strategies to modulate the tumour immune microenvironment for systemic anti-tumour immunity
British Journal of Cancer (2022)
-
Toll-like receptors form different complexes with UNC93B1
Nature Structural & Molecular Biology (2021)
-
Cryo-EM structures of Toll-like receptors in complex with UNC93B1
Nature Structural & Molecular Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.