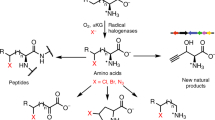
Abstract
Living systems can generate an enormous range of cellular functions, from mechanical infrastructure and signalling networks to enzymatic catalysis and information storage, using a notably limited set of chemical functional groups. This observation is especially notable when compared to the breadth of functional groups used as the basis for similar functions in synthetically derived small molecules and materials. The relatively small cross-section between biological and synthetic reactivity space forms the foundation for the development of bioorthogonal chemistry, in which the absence of a pair of reactive functional groups within the cell allows for a selective in situ reaction1,2,3,4. However, biologically ‘rare’ functional groups, such as the fluoro5, chloro6,7, bromo7,8, phosphonate9, enediyne10,11, cyano12, diazo13, alkene14 and alkyne15,16,17 groups, continue to be discovered in natural products made by plants, fungi and microorganisms, which offers a potential route to genetically encode the endogenous biosynthesis of bioorthogonal reagents within living organisms. In particular, the terminal alkyne has found broad utility via the Cu(i)-catalysed azide-alkyne cycloaddition ‘click’ reaction18. Here we report the discovery and characterization of a unique pathway to produce a terminal alkyne-containing amino acid in the bacterium Streptomyces cattleya. We found that l-lysine undergoes an unexpected reaction sequence that includes halogenation, oxidative C–C bond cleavage and triple bond formation through a putative allene intermediate. This pathway offers the potential for de novo cellular production of halo-, alkene- and alkyne-labelled proteins and natural products from glucose for a variety of downstream applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Accession codes for genes and proteins in this study are provided in Supplementary Tables. Source data files for figures are provided. Datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Prescher, J. A. & Bertozzi, C. R. Chemistry in living systems. Nat. Chem. Biol. 1, 13–21 (2005).
Li, J. & Chen, P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 12, 129–137 (2016).
Chin, J. W. et al. An expanded eukaryotic genetic code. Science 301, 964–967 (2003).
Wright, T. H. et al. Posttranslational mutagenesis: a chemical strategy for exploring protein side-chain diversity. Science 354, aag1465 (2016).
O’Hagan, D., Schaffrath, C., Cobb, S. L., Hamilton, J. T. G. & Murphy, C. D. Biochemistry: biosynthesis of an organofluorine molecule. Nature 416, 279 (2002).
Vaillancourt, F. H., Yeh, E., Vosburg, D. A., O’Connor, S. E. & Walsh, C. T. Cryptic chlorination by a non-haem iron enzyme during cyclopropyl amino acid biosynthesis. Nature 436, 1191–1194 (2005).
Agarwal, V. et al. Enzymatic halogenation and dehalogenation reactions: Pervasive and mechanistically diverse. Chem. Rev. 117, 5619–5674 (2017).
Agarwal, V. et al. Biosynthesis of polybrominated aromatic organic compounds by marine bacteria. Nat. Chem. Biol. 10, 640–647 (2014).
Cicchillo, R. M. et al. An unusual carbon–carbon bond cleavage reaction during phosphinothricin biosynthesis. Nature 459, 871–874 (2009).
Liu, W., Christenson, S. D., Standage, S. & Shen, B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 297, 1170–1173 (2002).
Ahlert, J. et al. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 297, 1173–1176 (2002).
Jensen, N. B. et al. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat. Commun. 2, 273 (2011).
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat. Chem. Biol. 12, 73–75 (2016).
Rui, Z. et al. Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase. Proc. Natl Acad. Sci. USA 111, 18237–18242 (2014).
Zhu, X., Liu, J. & Zhang, W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat. Chem. Biol. 11, 115–120 (2015).
Haritos, V. S. et al. The convergent evolution of defensive polyacetylenic fatty acid biosynthesis genes in soldier beetles. Nat. Commun. 3, 1150 (2012).
Scrimgeour, C. M. Natural acetylenic and olefinic compounds, excluding marine natural products. Aliphatic Relat. Nat. Prod. Chem. 2, 1–19 (1979).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Minto, R. E. & Blacklock, B. J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 47, 233–306 (2008).
Potgieter, H. C., Vermeulen, N. M. J., Potgieter, D. J. J. & Strauss, H. F. A toxic amino acid, 2(S)3(R)-2-amino-3-hydroxypent-4-ynoic acid from the fungus Sclerotium rolfsii. Phytochemistry 16, 1757–1759 (1977).
Sanada, M., Miyano, T. & Iwadare, S. β-Ethynylserine, an antimetabolite of L-threonine, from Streptomyces cattleya. J. Antibiot. (Tokyo) 39, 304–305 (1986).
Scannell, J. P., Pruess, D. L., Demny, T. C., Weiss, F. & Williams, T. Antimetabolites produced by microorganisms. II. L-2-amino-4-pentynoic acid. J. Antibiot. (Tokyo) 24, 239–244 (1971).
Truong, F., Yoo, T. H., Lampo, T. J. & Tirrell, D. A. Two-strain, cell-selective protein labeling in mixed bacterial cultures. J. Am. Chem. Soc. 134, 8551–8556 (2012).
Lang, K. & Chin, J. W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 114, 4764–4806 (2014).
Zhu, X., Su, M., Manickam, K. & Zhang, W. Bacterial genome mining of enzymatic tools for alkyne biosynthesis. ACS Chem. Biol. 10, 2785–2793 (2015).
Shin-Ichi, H. Amino acids from mushrooms. Prog. Chem. Org. Nat. Prod. 59,117–140 (1992).
Tautenhahn, R., Patti, G. J., Rinehart, D. & Siuzdak, G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal. Chem. 84, 5035–5039 (2012).
Wong, S. D. et al. Elucidation of the Fe(IV)=O intermediate in the catalytic cycle of the halogenase SyrB2. Nature 499, 320–323 (2013).
Brzović, P., Holbrook, E. L., Greene, R. C. & Dunn, M. F. Reaction mechanism of Escherichia coli cystathionine γ-synthase: direct evidence for a pyridoxamine derivative of vinylglyoxylate as a key intermediate in pyridoxal phosphate dependent γ-elimination and γ-replacement reactions. Biochemistry 29, 442–451 (1990).
Sun, Q. et al. Structural basis for the inhibition mechanism of human cystathionine γ-lyase, an enzyme responsible for the production of H2S. J. Biol. Chem. 284, 3076–3085 (2009).
Marcotte, P. & Walsh, C. Vinylglycine and proparglyglycine: complementary suicide substrates for L-amino acid oxidase and D-amino acid oxidase. Biochemistry 15, 3070–3076 (1976).
Abeles, R. H. & Walsh, C. T. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase, in vitro and in vivo, by propargylglycine. J. Am. Chem. Soc. 95, 6124–6125 (1973).
Cline, M. S. et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protocols 2, 2366–2382 (2007).
Merkx, R. et al. Scalable synthesis of γ-thiolysine starting from lysine and a side by side comparison with δ-thiolysine in non-enzymatic ubiquitination. Chem. Sci. (Camb.) 4, 4494 (2013).
Schwarzenbacher, R. et al. Structure of the Chlamydia protein CADD reveals a redox enzyme that modulates host cell apoptosis. J. Biol. Chem. 279, 29320–29324 (2004).
Guy, J. E. et al. Remote control of regioselectivity in acyl-acyl carrier protein-desaturases. Proc. Natl Acad. Sci. USA 108, 16594–16599 (2011).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protocols 10, 845–858 (2015).
McCune, C. D. et al. Synthesis and deployment of an elusive fluorovinyl cation equivalent: Access to quaternary α-(1′-fluoro)vinyl amino acids as potential PLP enzyme inactivators. J. Am. Chem. Soc. 139, 14077–14089 (2017).
Craig, R. & Beavis, R. C. TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20, 1466–1467 (2004).
Acknowledgements
We thank W. Zhang, D. Nomura and D. Berkowitz for discussions and advice. J.A.M. acknowledges the support of a UC Berkeley Chancellor’s Fellowship, Howard Hughes Medical Institute Gilliam Fellowship, and National Institutes of Health NRSA Training Grant (1 T32 GMO66698). M.E.N. acknowledges the support of a National Science Foundation Graduate Research Fellowship. This work was funded by generous support from the National Science Foundation (CHE-1710588). The College of Chemistry NMR Facility at U.C. Berkeley is supported in part by the National Institutes of Health (1S10RR023679-01 and S10 RR16634-01).
Reviewer information
Nature thanks Gonçalo Bernardes, Rebecca Goss and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.A.M. carried out gene disruption and in vivo reconstitution experiments with assistance from M.C.I. J.A.M. and M.E.N. carried out experiments for comparative metabolomics and enzyme characterization. J.G.P. planned and carried out NMR experiments. J.A.M., M.E.N., C.-I.L. and M.C.Y.C. planned experiments. J.A.M., M.E.N. and M.C.Y.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Genetic and bioinformatic analysis of terminal-alkyne amino acid production in S. cattleya.
a, Four fatty acid desaturases were identified in the genome of S. cattleya and targeted for deletion. Per cent identity (%ID) and per cent similarity (%S) compared to the closest sequenced homologue are shown with the host in brackets. Number of amino acids in protein also tabulated. SCAT_0184 is adjacent to type I polyketide synthase and may be part of a biosynthetic cluster. SCAT_p1525 may also have a metabolic role, although its genomic context is not highly informative. SCAT_2136 and SCAT_4823 are clustered with proteins thought to be involved in primary metabolism and rRNA transcription. We were unable to identify any deletion strains of SCAT_2136, suggesting that SCAT_2136 may be an essential fatty acid desaturase. Knockouts of SCAT_0184, SCAT_4823, and SCAT_p1525 were found to still produce βes by CuAAC derivatization with 3-azido-7-hydroxy-coumarin followed by liquid chromatography and quadrupole time of flight (LC–QTOF) monitoring of the βes-coumarin CuAAC product (βes-Cou). The reaction in which Cu(i) is omitted provides a negative control because Cu(i) is required for the cycloaddition to occur. b, Extracted ion chromatograms of the culture supernatant of S. catenulae with and without CuAAC derivatization, monitoring either the Pra-coumarin adduct (Pra-Cou; m/z = 317.0880) or Pra (m/z = 114.0550) compared to a synthetic Pra standard using LC–QTOF. Chromatograms shown are representative of at least three independent experimental replicates. c, We generated a list of approximately 650 gene clusters (>2 genes) that were conserved between both species. Clusters were generated using Cytoscape33. Nodes are individual genes and edges connect genes that are within two protein-coding regions of one another. Gene clusters comprise of connected nodes. Connected grey nodes are gene clusters also found in any of the other 26 Streptomyces spp. used in the analysis. Connected red nodes are gene clusters found to be unique between S. cattleya and S. catenulae. We then curated this list by removing gene clusters that are more closely related between S. cattleya and 26 other Streptomyces spp., narrowing down the number of conserved unique clusters to just 4. One of these unique clusters contains member nodes WP_014151494.1, WP_014151495.1, WP_014151496.1 and WP_014151497.1, which composes the putative bes gene cluster (indicated with a red arrow).
Extended Data Fig. 2 βes biosynthetic clusters in Streptomyces spp. and production phenotypes of wild-type and knockout strains.
a, Genome clusters and genomic context of βes/Pra and Pra biosynthetic clusters (coloured) in S. cattleya, S. sp. NRRL S-1448, S. alboverticillatus, S. catenulae, S. achromogenes, S. lavenduligriseus, and S. sp. NRRL S-31. The βes/Pra core cluster contains besE and besF in addition to besA–besD, whereas the Pra core cluster contains only besA–besD. The S. alboverticillatus besF is predicted on the basis of partial sequencing of the gene from the incomplete genome assembly. b, Characterization of Streptomyces spp. containing the βes/Pra and Pra biosynthetic clusters for their ability to produce terminal-alkyne amino acids. The presence of βes and Pra were analysed by derivatization with 3-azido-7-hydroxy-coumarin by CuAAC and monitoring of either the βes-coumarin adduct (βes-Cou; m/z = 333.0831) or the Pra-coumarin adduct (Pra-Cou; m/z = 317.0880) in the presence and absence of Cu(i) using LC–QTOF. The reaction in which Cu(i) was omitted provided a negative control because it is required for the cycloaddition to occur. Extracted ion chromatograms of S. cattleya and S. sp. NRRL S-1448 supernatants showing the production of βes (βes-Cou; m/z = 333.0831) (top). Extracted ion chromatograms of S. cattleya, S. achromogenes, S. sp. NRRL S-31, S. sp. NRRL S-1448 and S. lavenduligriseus supernatants show the production of Pra (Pra-Cou; m/z = 317.0880). S. lavenduligriseus was cultured but not found to produce Pra. Attempts to amplify genes in the βes cluster failed, which suggests that this particular strain may have lost the bes genes. Chromatograms shown are representative of at least three independent experimental replicates. c, S. cattleya knockout strains were complemented with the corresponding wild-type gene expressed from the ErmEp∗ promoter by insertion into the phage attachment site in the genome. Knockout and complementation strains were cultured for 7 d. The supernatants were derivatized with 3-azido-7-hydroxy-coumarin by CuAAC and analysed for the βes-coumarin adduct (βes-Cou; m/z = 333.0831) by LC–QTOF. Only the besB complementation strain appeared to recover βes production, possibly owing to polar effects related to ordering of the genes in the putative operon (besD–besC–besB). Chromatograms shown are representative of at least three independent experimental replicates.
Extended Data Fig. 3 Identification of pathway intermediates using comparative metabolomics and Bes proteins used for in vitro biochemical experiments.
a, Extracted ion chromatograms of pathway intermediates identified using comparative metabolomics from S. cattleya pellet extracts for 4-Cl-lysine (2, m/z = 181.0738), 4-Cl-allylglycine (3, m/z = 150.0316), propargylglycine (4, m/z = 114.0550), γ-Glu-Pra (5, m/z = 243.0975) and γ-Glu-Bes (6, m/z = 259.0925). Chromatograms shown are representative of at least five independent experimental replicates. b, SDS–PAGE of purified enzymes used for biochemical assays and in vitro reconstitution of pathway. 1, His10–BesA; 2, His6MBP–BesB; 3, BesC; 4, BesD; 5, His10–BesE. All proteins are from S. cattleya except for BesB, which is from S. sp. NRRL S-1448. c, SDS–PAGE of His6MBP–BesB solubility screen from S. sp. NRRL S-1448. BesB from S. cattleya was found to be highly insoluble, therefore the orthologue S. sp. NRRL S-1448 was cloned and purified. Lanes correspond to each cell line tested (1, pre-induction; 2, post-induction; 3, elution from Ni-NTA column). The highest soluble expression was observed in E. coli BL21(DE3)-Star.
Extended Data Fig. 4 Characterization of the BesD halogenase product and side products.
a, BesD is a putative Fe/αKG-dependent halogenase with a conserved HXG motif rather than the expected HXD/E motif for hydroxylases. b, BesD activity requires l-lysine, Cl–, Fe(II) and αKG (4-Cl-lysine, 2; m/z = 181.0738). Chromatograms shown are representative of at least three independent experimental replicates. c, LC–QTOF traces monitoring production of 4-Cl-lysine (2, m/z = 181.0738) and 4-OH-lysine (m/z = 163.1077) in reactions with no enzyme, BesD or the BesD(G139D) mutant. Chromatograms shown are representative of three independent experimental replicates. As previously discussed, a low level of 4-OH-lysine is expected from direct hydroxylation of l-lysine by wild-type BesD, as has been commonly observed in other members of the αKG/Fe-dependent halogenase family. d, Circular dichroism (CD) spectra of wild-type BesD compared to the G139D mutant suggest that BesD(G139D) retains a similar fold or secondary structure content (MRE, molar residue ellipticity). Spectra shown are representative of three independent experimental replicates. e, BesD was incubated with fully 15N- and 13C-labelled l-lysine, Fe and αKG for 20 min before being quenched with methanolic HCl (3M), to yield the [15N2,13C6]-4-Cl-lysine methyl ester product. Chloride was provided as NaCl in the enzyme storage buffer. We found that derivatization was necessary to avoid intramolecular lactam formation. The derivatized BesD product was then isolated by HPLC and characterized. Extracted ion chromatograms of the [15N2, 13C6]-4-Cl-lysine (m/z = 203.1037) produced by BesD are shown. Chromatograms shown are representative of three independent experimental replicates. f, The mass spectrum shows the characteristic Cl isotope pattern for [15N2,13C6]-4-Cl-lysine methyl ester produced by BesD and is representative of three independent experimental replicates. g, Two-dimensional 1H-13C constant-time heteronuclear single quantum correlation (CT-HSQC) was used to confirm assignment of Cα and Cε of the [15N2,13C6]-4-Cl-lysine methyl ester produced by BesD. In this experiment carbons with two neighbours (red) have the opposite phase of carbons with one or three neighbours (blue). h, Two-dimensional 1H-13C HCCH correlation spectroscopy (COSY) was used to assign connectivity of carbons of the [15N2,13C6]-4-Cl-lysine methyl ester produced by BesD.
Extended Data Fig. 5 Characterization of the 4-Cl-lysine (2) shunt pathway.
a, 4-Cl-lysine (2) is proposed to undergo an intramolecular nucleophilic attack to form 2-amino-4-propylamine-γ-lactone with an estimated t1/2 = 1 h34. The γ-lactone is also unstable and can form either the 2-amino-4-hydroxy-ε-lactam or 4-OH-lysine. 4-OH-lysine could also arise from direct hydroxylation of l-lysine by BesD, as a low level of hydroxylation is observed in other members of the Fe/αKG-dependent halogenase family. b, Integrated extracted ion chromatograms for each of the observed products at pH 6, 7 and 8, following removal of BesD by filtration (Pall Nanosep filter, 10 kDa MWCO). Data are plotted relative to lysine, which is taken to be constant following enzyme removal. After 1 h, 4-Cl-lysine decreases, whereas the lactam, lactone and 4-OH-lysine products increase in abundance. c, Extracted ion chromatograms of the decomposition products observed upon halogenation of l-lysine by BesD by LC–QTOF. Lysine, m/z = 147.1128; 4-Cl-lysine (2), m/z = 181.0738; ε-lactam (2a)/γ-lactone (2b), m/z = 145.0972; 4-OH-lysine (2c), m/z = 163.1077. Chromatograms shown are representative of at least three independent experimental replicates. d, To obtain the lactam structure, BesD was incubated with fully 15N- and 13C-labelled l-lysine, Fe, and αKG for 16 h before being quenched with 1% (v/v) formic acid in methanol to yield the [15N2,13C6]-lactam as the major product. Chloride was provided as NaCl in the enzyme storage buffer. NMR analysis of the ε-lactam was carried out after HPLC purification. Two-dimensional 1H-13C CT-HSQC with a 13.6-ms 13C evolution period was used to confirm assignment of Cα and Cε. In this experiment, carbons with two neighbours (red) have the opposite phase of carbons with one or three neighbours (blue). e, 1H-13C HCCH COSY was used to assign backbone connectivity of carbons for the lactam. f, The 1H-15N HSQC spectrum confirms the presence of the amide in the lactam structure, with a reported chemical shift typical of amides (δ 6–9 p.p.m.). g, The 2D 1H-13C HNCO spectrum confirms that the epsilon N is adjacent to a carbonyl species. h, The 2D 1H-13C HNCA spectrum confirms that the epsilon N is coupled to Cε, forming a cyclic structure.
Extended Data Fig. 6 Detection of ammonia as a co-product of the BesC reaction.
a, BesC shows homology to non-haem diiron proteins such as the Chlamydia protein associated with death domains (CADD, 34% sequence similarity35) and stearoyl-ACP desaturase (26% sequence similarity36). Overlay of the predicted homology model of BesC (blue) with the CADD crystal structure (green)37 reveals six conserved putative Fe-binding residues in the active site. Putative iron ligands shown in brown. Homology model was generated using Phyre 2. b, BesC catalyses formation of 4-Cl-allylglycine from 4-Cl-lysine and requires Fe(II) as a cofactor (4-Cl-allylglycine, 3; m/z = 150.0316). Chromatograms shown are representative of at least three independent experimental replicates. c, The BesC reaction is run as a coupled reaction with BesD, starting from l-lysine, αKG and chloride. To detect the ammonia co-product, we used glutamate dehydrogenase (GDH), which produces glutamate from ammonia and αKG with concurrent oxidation of NADPH. d, The release of ammonia from 4-Cl-lysine was monitored through incorporation of the nitrogen from the 15N-labelled lysine substrate into glutamate using LC–QTOF. Positive ionization mass spectrum showing formation of 14N-glutamate or 15N-l-glutamate when unlabelled l-lysine (left) or [15N2,13C6]-l-lysine (right) are used as the substrate, respectively. Spectra shown are representative of three independent experimental replicates. e, BesC is also competent to react with l-lysine directly to produce allylglycine in vitro. LC–QTOF traces show allylglycine produced by BesC compared to a synthetic standard (Alg, m/z = 116.0706). Chromatograms shown are representative of at least three independent experimental replicates. f, Consumption of NADPH was observed spectrophotometrically. Reactions contained BesC, BesD, lysine, αKG, sodium ascorbate, Fe(II) and chloride, as indicated. After 1 h, NADPH and glutamate dehydrogenase were added and NADPH consumption was monitored by A340. g, Standard curves constructed by integrating extracted ion counts for the relevant species by LC–QTOF. R2 value shown was determined by ordinary least squares. h, Quantification by LC–QTOF showing stoichiometric ammonia release measured as glutamate (Glu) compared total alkene product (4-Cl-allylglycine, Cl-Alg; allylglycine, Alg) in a coupled assay of BesD and BesC with l-lysine using l-glutamate dehydrogenase. Data are mean ± s.d. (n = 3 technical replicates).
Extended Data Fig. 7 Detection of formaldehyde as a co-product of the BesC reaction.
The BesC reaction was run as a coupled reaction with BesD starting from l-lysine, αKG and chloride. a, To derivatize the putative formaldehyde co-product, we performed the enzymatic reaction in the presence of Fluoral-P. The release of formaldehyde from 4-Cl-lysine was monitored through incorporation of the carbon from 13C-labelled lysine substrate into 3,5-diacetyl-1,4-dihydro-2,6-lutidine (DDL). b, Positive ionization mass spectrum showing formation of 12C-DDL or 13C-DDL when unlabelled l-lysine (left) or [15N2,13C6]-l-lysine (right) is used as the substrate, respectively. Spectra shown are representative of three independent experimental replicates. c, Standard curves for the relevant species were generated by integration of the extracted ion chromatogram from LC–TOF (Cl-Alg, Alg) or by fluorescence quantification (formaldehyde). R2 value shown was determined by ordinary least squares. d, Quantification of formaldehyde produced from Fluoral-P reaction with 5 mM l-lysine and purified BesD, BesC, or both BesC and BesD. The resulting product was quantified by fluorescence and compared to alkene product formation measured by LC–QTOF and found to be stoichiometric. Data are mean ± s.d. (n = 3 technical replicates).
Extended Data Fig. 8 In vitro characterization of BesB and proposed mechanism of terminal alkyne formation.
a, LC–QTOF analysis of in vitro reactions containing BesB, PLP and substrate. When the substrate was 4-Cl-Alg or 4-Br-Alg, Pra (4, m/z = 114.0550) was observed. However, no Pra is observed if allylglycine is used as a substrate. Chromatograms shown are representative of at least three independent experimental replicates. b, In vitro reconstitution of Pra (4, m/z = 114.0550) formation from l-lysine with purified BesB, BesC, and BesD and Fe(II), αKG, chloride, PLP and ascorbate, monitored by LC–QTOF. Chromatograms shown are representative of at least three independent experimental replicates. c, A mechanistic model for BesB is proposed in analogy to cystathionine γ-synthase and related PLP mechanisms that form allene-intermediates38. In the first step, the external 4-Cl-allylglycine-aldimine is formed. Deprotonation at the Cα-position yields the 4-Cl-allylglycine-carbanion or quinonoid or intermediate. Deprotonation at the Cβ-position produces the 4-Cl-allylglycine-ketamine intermediate. Elimination of chloride can then occur upon a 90° rotation to break planarity or aromatization to form a proposed terminal-allene intermediate. Work using Pra as a mechanistic inhibitor of PLP enzymes shows that isomerization between the terminal alkyne and allene can occur30,31,32. At this time, we suggest that the reverse can occur to convert the PLP-bound allene to the terminal alkyne, yielding a PLP-bound Pra intermediate. The Cδ deprotonation to initiate this step is facilitated by overlap of the Cδ–H bond with the conjugated Cβ–Cγ and PMP–imine π-bonds. Subsequent steps then allow regeneration of the external aldimine with Pra, which is then released from the cofactor.
Extended Data Fig. 9 In vitro and in vivo characterization of BesA and BesE.
a, Relative abundance of the γ-Glu-Pra (5, m/z = 243.0975) and γ-Glu-βes (6; m/z = 259.0925) dipeptides in S. cattleya wild-type and pathway knockout strains (n = 5 biological replicates; dot shows mean; line shows median; box edges show 25th and 75th percentiles; error bars show 95th percentile). b, Fragmentation of γ-Glu-Pra (left) and γ-Glu-βes (right) in negative ionization mode by liquid chromatography with triple quadrupole mass spectrometry shows fragments characteristic of γ-glutamyl-dipeptides (m/z = 128.0). Spectra shown are representative of at least three independent experimental replicates. c, LC–MS traces for enzymatically synthesized γ-Glu-Pra (5, m/z = 243.0975) by either γ-GT or BesA and LC–QTOF traces for enzymatically synthesized γ-Glu-βes (6, m/z = 259.0925) by either γ-GT plus BesE or BesA plus BesE. Chromatograms shown are representative of at least three independent experimental replicates. d, Steady-state kinetic analysis for BesA reactions between l-glutamate and various l-amino acid partners (Nva, norvaline). Each bar represents the kcat/KM value for the corresponding amino acid calculated from the individual kinetic terms (kcat and KM) obtained from nonlinear curve fitting of data to the Michaelis–Menten equation. Data shown are mean ± s.e.m. (n = 3 technical replicates) obtained by propagation. Only reactions between l-glutamate and l-propargylglycine or l-cysteine showed any detectable activity, which suggests that BesA is relatively selective and that the residual Cys activity may be a product of its evolutionary history. e, Steady-state kinetic analysis of BesA with 10 mM glutamate and l-propargylglycine or l-cysteine as substrates. Data are mean ± s.d. (n = 3 technical replicates). Table contains kcat, KM, and kcat/KM calculated by nonlinear curve fitting to the Michaelis–Menten equation. Data are mean ± s.e. Error in kcat/KM is obtained by propagation from the individual kinetic terms. f, Top, extracted ion chromatograms for γ-Glu-Pra in reactions containing a combination of BesA, BesE and cofactors (γ-Glu-Pra, 5; m/z = 243.0975). Bottom, extracted ion chromatograms for γ-Glu-βes in reactions containing a combination of BesA, BesE and cofactors (γ-Glu-βes, 6; m/z = 259.0925). Chromatograms shown are representative of at least three independent experimental replicates.
Extended Data Fig. 10 In vivo production of amino acids and alkyne-labelled proteins using genes from the bes gene cluster in E. coli.
a, BesB functional expression was tested in different medium conditions as it is the limiting factor in the biosynthesis of Pra. Expression was tested by analysing production of Pra (4, m/z = 114.550) by LC–QTOF in E. coli BL21 Star (DE3) pSV272.1-MBP-BesB cultures fed with substrate (1 mM 4-Cl-allylglycine, 3). Cells were collected after 24 h of growth. Chromatograms shown are representative of three independent experimental replicates. b, Optimization of BesB functional expression with co-expression of various E. coli protein chaperones. Production of Pra (4, m/z = 114.550) was monitored by LC–QTOF in E. coli BL21 Star (DE3) pSV272.1-MBP-BesB cultures fed with substrate (1 mM 4-Cl-allylglycine, 3). Cells were collected after 24 h of growth in TB. Chromatograms shown are representative of three independent experimental replicates. c, SDS–PAGE gel from Fig. 4d showing Pra incorporation into the E. coli proteome by CuAAC derivatization, stained with Coomasie dye to visualize total protein loading in each lane. Gel shown is representative of six independent experimental replicates. d, Additional controls for the SDS–PAGE gel from Fig. 4d showing that successful derivatization with Tamra-azide requires endogenous Pra production, PraRS and Cu. In this experiment, E. coli B834(DE3) pPra praGro was cultured for 2 d along with the respective empty vector controls and no-Cu controls. The cell lysates were then derivatized with Tamra-azide dye by CuAAC before analysis by SDS–PAGE under fluorescence mode. Reactions without Cu act a negative control because Cu is required for CuAAC. Ladder consists of 70-kDa marker that can be visualized by fluorescence overlaid with the Coomassie-stained ladder from the same gel for reference. Gel shown is representative of six independent experimental replicates. e, Total protein was extracted from cell pellets of E. coli B834(DE3) pPra PraGro. The extracted protein was subject to tryptic digest and bottom-up proteomic analysis using an using a Thermo LC-Q Exactive and spectra were analysed using X! Tandem39. Representative high-resolution MS/MS spectra of peptides (GroEL, showing Pra replacing Met from E. coli B834(DE3) pPra PraGro are shown (EIELEDKFENMGAQ(Pra)VK, GroL, WP_000729117; LF(Pra)DEQIEYILK, BesC, WP_080628534; GG(Pra)LQC∗VPHTSWDK, BesD, WP_016975823). The spectrum above the axis gives peptide-spectrum matches to sequences with Pra substituted for Met; the spectrum below the axis shows the corresponding Met-containing native peptide (C∗ = carbamidomethyl derivative from iodoacetamide capping). Spectra shown are representative of two independent experimental replicates.
Supplementary information
Supplementary Information
The file contains supplementary methods for construction of plasmids and strains, expression and purification of proteins, purification and analysis of compounds, in vitro assays, as well as a list of commercial material sources. Supplementary tables are included for strains, plasmids, oligonucleotides, and synthetic gene sequences used for this study. Accession IDs for Bes proteins are also tabulated
Supplementary Data
The product of the BesD halogenase was derivatized to prevent intramolecular cyclization. This data file contains the raw data for the 1D-NMR (1H and 13C-NMR, Figure S1) and 2D-NMR (CT- HSQC and HCCH-COSY, Extended Data Figure 4) for the resulting [13C6, 15N2]-4-Cl-lysine methyl ester
Source data
Rights and permissions
About this article
Cite this article
Marchand, J.A., Neugebauer, M.E., Ing, M.C. et al. Discovery of a pathway for terminal-alkyne amino acid biosynthesis. Nature 567, 420–424 (2019). https://doi.org/10.1038/s41586-019-1020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1020-y
This article is cited by
-
Accelerating the discovery of alkyl halide-derived natural products using halide depletion
Nature Chemistry (2024)
-
Characterization of an efficient N-oxygenase from Saccharothrix sp. and its application in the synthesis of azomycin
Biotechnology for Biofuels and Bioproducts (2023)
-
A platform for distributed production of synthetic nitrated proteins in live bacteria
Nature Chemical Biology (2023)
-
The structural and functional investigation into an unusual nitrile synthase
Nature Communications (2023)
-
THRONCAT: metabolic labeling of newly synthesized proteins using a bioorthogonal threonine analog
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.