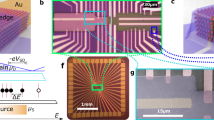
Abstract
Accessing the intrinsic functionality of molecules for electronic applications1,2,3, light emission4 or sensing5 requires reliable electrical contacts to those molecules. A self-assembled monolayer (SAM) sandwich architecture6 is advantageous for technological applications, but requires a non-destructive, top-contact fabrication method. Various approaches ranging from direct metal evaporation6 over poly(3,4-ethylenedioxythiophene) polystyrene sulfonate7 (PEDOT:PSS) or graphene8 interlayers to metal transfer printing9 have been proposed. Nevertheless, it has not yet been possible to fabricate SAM-based devices without compromising film integrity, intrinsic functionality or mass-fabrication compatibility. Here we develop a top-contact approach to SAM-based devices that simultaneously addresses all these issues, by exploiting the fact that a metallic nanoparticle can provide a reliable electrical contact to individual molecules10. Our fabrication route involves first the conformal and non-destructive deposition of a layer of metallic nanoparticles directly onto the SAM (itself laterally constrained within circular pores in a dielectric matrix, with diameters ranging from 60 nanometres to 70 micrometres), and then the reinforcement of this top contact by direct metal evaporation. This approach enables the fabrication of thousands of identical, ambient-stable metal–molecule–metal devices. Systematic variation of the composition of the SAM demonstrates that the intrinsic molecular properties are not affected by the nanoparticle layer and subsequent top metallization. Our concept is generic to densely packed layers of molecules equipped with two anchor groups, and provides a route to the large-scale integration of molecular compounds into solid-state devices that can be scaled down to the single-molecule level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Elbing, M. et al. A single-molecule diode. Proc. Natl Acad. Sci. USA 102, 8815–8820 (2005).
Chen, J., Reed, M. A., Rawlett, A. M. & Tour, J. M. Large on-off ratios and negative differential resistance in a molecular electronic device. Science 286, 1550–1552 (1999).
Schwarz, F. et al. Field-induced conductance switching by charge-state alternation in organometallic single-molecule junctions. Nat. Nanotechnol. 11, 170–176 (2016).
Marquardt, C. W. et al. Electroluminescence from a single nanotube–molecule–nanotube junction. Nat. Nanotechnol. 5, 863–867 (2010).
Ponce, J. et al. Effect of metal complexation on the conductance of single-molecular wires measured at room temperature. J. Am. Chem. Soc. 136, 8314–8322 (2014).
Zhou, C., Deshpande, M. R., Reed, M. A., Jones, L. & Tour, J. M. Nanoscale metal/self-assembled monolayer/metal heterostructures. Appl. Phys. Lett. 71, 611–613 (1997).
Akkerman, H. B., Blom, P. W. M., de Leeuw, D. M. & de Boer, B. Towards molecular electronics with large-area molecular junctions. Nature 441, 69–72 (2006).
Wang, G., Kim, Y., Choe, M., Kim, T.-W. & Lee, T. A new approach for molecular electronic junctions with a multilayer graphene electrode. Adv. Mater. 23, 755–760 (2011).
Loo, Y.-L., Lang, D. V., Rogers, J. A. & Hsu, J. W. P. Electrical contacts to molecular layers by nanotransfer printing. Nano Lett. 3, 913–917 (2003).
Cui, X. D. et al. Reproducible measurement of single-molecule conductivity. Science 294, 571–574 (2001).
Nijhuis, C. A., Reus, W. F., Barber, J. R., Dickey, M. D. & Whitesides, G. M. Charge transport and rectification in arrays of SAM-based tunneling junctions. Nano Lett. 10, 3611–3619 (2010).
Jeong, H. et al. A new approach for high-yield metal–molecule–metal junctions by direct metal transfer method. Nanotechnology 26, 025601 (2015).
Lee, T. et al. Comparison of electronic transport characterization methods for alkanethiol self-assembled monolayers. J. Phys. Chem. B 108, 8742–8750 (2004).
Kim, T.-W., Wang, G., Lee, H. & Lee, T. Statistical analysis of electronic properties of alkanethiols in metal–molecule–metal junctions. Nanotechnology 18, 315204 (2007).
Neuhausen, A. B., Hosseini, A., Sulpizio, J. A., Chidsey, C. E. D. & Goldhaber-Gordon, D. Molecular junctions of self-assembled monolayers with conducting polymer contacts. ACS Nano 6, 9920–9931 (2012).
Lee, S. et al. Self-assembled monolayers on Pt(111): molecular packing structure and strain effects observed by scanning tunneling microscopy. J. Am. Chem. Soc. 128, 5745–5750 (2006).
Beebe, J. M., Engelkes, V. B., Miller, L. L. & Frisbie, C. D. Contact resistance in metal–molecule–metal junctions based on aliphatic SAMs: effects of surface linker and metal work function. J. Am. Chem. Soc. 124, 11268–11269 (2002).
Puebla-Hellmann, G., Mayor, M. & Lörtscher, E. Ultraflat nanopores for wafer-scale molecular-electronic applications. In 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO) 1197–1201 (IEEE, 2015).
Akkerman, H. B. et al. Self-assembled-monolayer formation of long alkanedithiols in molecular junctions. Small 4, 100–104 (2008).
Jia, J. et al. Lying-down to standing-up transitions in self assembly of butanedithiol monolayers on gold and substitutional assembly by octanethiols. J. Phys. Chem. C 117, 4625–4631 (2013).
Chah, S., Fendler, J. H. & Yi, J. In-situ analysis of stepwise self-assembled 1,6-hexanedithiol multilayers by surface plasmon resonance measurements. Chem. Commun. 18, 2094–2095 (2002).
Engelkes, V. B., Beebe, J. M. & Frisbie, C. D. Length-dependent transport in molecular junctions based on SAMs of alkanethiols and alkanedithiols: effect of metal work function and applied bias on tunneling efficiency and contact resistance. J. Am. Chem. Soc. 126, 14287–14296 (2004).
Lörtscher, E., Mayor, M. & Puebla-Hellmann, G. Contacting molecular components. US patent application 20180062076 (2018).
Sur, U. K. & Lakshminarayanan, V. Existence of a hydrophobic gap at the alkanethiol SAM-water interface: an interfacial capacitance study. J. Colloid Interface Sci. 254, 410–413 (2002).
Wang, W., Lee, T. & Reed, M. A. Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. B 68, 035416 (2003).
Wang, W., Lee, T., Kretzschmar, I. & Reed, M. A. Inelastic electron tunneling spectroscopy of an alkanedithiol self-assembled monolayer. Nano Lett. 4, 643–646 (2004).
Jeong, H. et al. Investigation of inelastic electron tunneling spectra of metal-molecule-metal junctions fabricated using direct metal transfer method. Appl. Phys. Lett. 106, 063110 (2015).
Simmons, J. G. Low-voltage current-voltage relationship of tunnel junctions. J. Appl. Phys. 34, 238–239 (1963).
Simeone, F. C. et al. Defining the value of injection current and effective electrical contact area for EGaIn-based molecular tunneling junctions. J. Am. Chem. Soc. 135, 18131–18144 (2013).
Morita, T. & Lindsay, S. Determination of single molecule conductances of alkanedithiols by conducting-atomic force microscopy with large gold nanoparticles. J. Am. Chem. Soc. 129, 7262–7263 (2007).
Wold, D. J., Haag, R., Rampi, M. A. & Frisbie, C. D. Distance dependence of electron tunneling through self-assembled monolayers measured by conducting probe atomic force microscopy: Unsaturated versus saturated molecular junctions. J. Phys. Chem. B 106, 2813–2816 (2002).
Akkerman, H. B. Large-area Molecular Junctions. PhD thesis, Univ. Groningen (2008).
Wang, G., Kim, T.-W., Lee, H. & Lee, T. Influence of metal-molecule contacts on decay coefficients and specific contact resistances in molecular junctions. Phys. Rev. B 76, 205320 (2007).
Lee, T., Wang, W. & Reed, M. A. Intrinsic electronic transport through alkanedithiol self-assembled monolayer. Jpn. J. Appl. Phys. 44, 523 (2005).
Park, S. et al. Flexible molecular-scale electronic devices. Nat. Nanotechnol. 7, 438–442 (2012).
Seo, S. et al. Solution-processed reduced graphene oxide films as electronic contacts for molecular monolayer junctions. Angew. Chem. Int. Ed. 51, 108–112 (2012).
Reus, W. F. et al. Statistical tools for analyzing measurements of charge transport. J. Phys. Chem. C 116, 6714–6733 (2012).
Jeong, H. et al. Statistical investigation of the length-dependent deviations in the electrical characteristics of molecular electronic junctions fabricated using the direct metal transfer method. J. Phys. Condens. Matter 28, 094003 (2016).
Acknowledgements
We acknowledge technical support from M. Tschudy, S. Reidt, M. Sousa, U. Drechsler, A. Zulji and M. Bürge, as well as strategic support from B. Michel, W. Riess and A. Curioni. This work was funded by the NCCR MSE and SNF NRP 62.
Author information
Authors and Affiliations
Contributions
G.P.-H. conceived, developed and performed device fabrication, performed data collection and analysis, and wrote the manuscript. K.V. and M.M. provided chemical support and commented on the manuscript. E.L. initiated and supervised the project, contributed to the experimental setups and data analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Device response classification.
a, Three example curves of an open circuit. b, Four example curves with different ratios of resistance at 50 mV and 300 mV bias. The top two (orange and blue lines) are categorized as linear, whereas the lower two (yellow and grey lines) are categorized as nonlinear.
Extended Data Fig. 2 Surface and nanoparticle film topology.
a, AFM scan of a 2.7-μm-diameter pore. b, The surface at the bottom of the pore with a roughness of 0.39 nm RMS. c, A similar pore after film growth and nanoparticle deposition. d, Scan of the surface inside the pore, showing circular particles.
Extended Data Fig. 3 Device cross sections.
a, TEM images of part of a C10 device lamella. Both the platinum bottom electrode and the gold top electrode are visible as bright layers, separated by the SAM on the left side and the SiNx dielectric on the right. The top electrode is warped in some regions. b, Zoom into a warped region: the bottom electrode, SAM region and top electrode are clearly visible, as well as the electron-deposited platinum protection layer for extraction of the lamella (the grainy area at the top of the image). c, d, High-resolution TEM images of two different areas of the SAM region. Although crystal lattices are visible, no 3-nm-sized features can be identified that would correspond to nanoparticles.
Extended Data Fig. 4 Element analysis.
Energy-dispersive X-ray spectrum of a region located approximately 2 nm above the SAM. Three elements can be identified: carbon, gold and copper.
Extended Data Fig. 5 Dependence of device characteristics on temperature and particle diameter.
a, Arrhenius plot for two devices (solid black, 790 nm diameter; dashed blue, 5.5 μm), showing a very slight temperature dependence. b, c, Preliminary results of current density for C8 and C10 monolayers with two different nanoparticle diameters: 3 nm (b) and 5 nm (c). The current density decreases for larger nanoparticles.
Extended Data Fig. 6 Short- and long-term stability.
a, Histogram of the current ratio between upward and downward sweeps at 0.5 V, with no substantial change between the two sweeps. b, Histogram of the current ratio between the initial sweep and the sweep taken 136 days later, at 0.5 V. A large part of the devices did not change, or changed by only a small amount.
Extended Data Fig. 7 Device scatter versus area.
A comparison of the full width half maximum (FWHM) current density deviation σJ against active device area for the literature quoted in Extended Data Table 1. We obtain similar or lower deviation than the literature samples over a considerably increased range in device area.
Extended Data Fig. 8 Additional data for C4, C6, C8 and C10 devices.
a–d, Histograms of I versus A. e–h, Device categorization for different values of A. i–l, Histograms of J versus A. The molecular length increases from left to right (a, e, i, C4; b, f, j, C6; c, g, k, C8; d, h, l, C10).
Rights and permissions
About this article
Cite this article
Puebla-Hellmann, G., Venkatesan, K., Mayor, M. et al. Metallic nanoparticle contacts for high-yield, ambient-stable molecular-monolayer devices. Nature 559, 232–235 (2018). https://doi.org/10.1038/s41586-018-0275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0275-z
This article is cited by
-
In-situ electro-responsive through-space coupling enabling foldamers as volatile memory elements
Nature Communications (2023)
-
Anomalous refinement and uniformization of grains in metallic thin films
Nano Research (2023)
-
Hybrid molecular graphene transistor as an operando and optoelectronic platform
Nature Communications (2023)
-
Printable logic circuits comprising self-assembled protein complexes
Nature Communications (2022)
-
CNT-molecule-CNT (1D-0D-1D) van der Waals integration ferroelectric memory with 1-nm2 junction area
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.