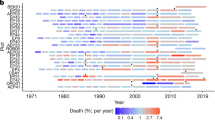
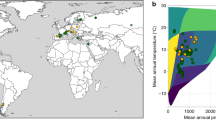
Abstract
Severe droughts have caused widespread tree mortality across many forest biomes with profound effects on the function of ecosystems and carbon balance. Climate change is expected to intensify regional-scale droughts, focusing attention on the physiological basis of drought-induced tree mortality. Recent work has shown that catastrophic failure of the plant hydraulic system is a principal mechanism involved in extensive crown death and tree mortality during drought, but the multi-dimensional response of trees to desiccation is complex. Here we focus on the current understanding of tree hydraulic performance under drought, the identification of physiological thresholds that precipitate mortality and the mechanisms of recovery after drought. Building on this, we discuss the potential application of hydraulic thresholds to process-based models that predict mortality.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bonan, G. B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Zhu, Z. et al. Greening of the Earth and its drivers. Nat. Clim. Change 6, 791–795 (2016).
Keenan, T. F. et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013).
Wenzel, S., Cox, P. M., Eyring, V. & Friedlingstein, P. Projected land photosynthesis constrained by changes in the seasonal cycle of atmospheric CO2. Nature 538, 499–501 (2016).
Reichstein, M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295 (2013).
Trenberth, K. E. et al. Global warming and changes in drought. Nat. Clim. Change 4, 17–22 (2014).
Tyree, M. T. & Zimmermann, M. H. Xylem Structure and the Ascent of Sap (Springer, New York, 2002).
Adams, H. D. et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1, 1285–1291 (2017).
Kursar, T. A. et al. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct. Ecol. 23, 93–102 (2009).
McDowell, N. et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178, 719–739 (2008). This study introduces a theoretical framework for understanding physiological mechanisms that underpin drought-induced mortality in trees.
Brodribb, T. J. & Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 149, 575–584 (2009). This study quantitatively links hydraulic failure thresholds to whole-plant mortality.
Urli, M. et al. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 33, 672–683 (2013).
Nardini, A., Battistuzzo, M. & Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 200, 322–329 (2013).
Venturas, M. D. et al. Chaparral shrub hydraulic traits, size, and life history types relate to species mortality during California’s historic drought of 2014. PLoS ONE 11, e0159145 (2016).
Anderegg, W. R. et al. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl Acad. Sci. USA 109, 233–237 (2012).
Davis, S. D. et al. Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. Am. J. Bot. 89, 820–828 (2002).
McDowell, N. G. et al. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 26, 523–532 (2011).
Duan, H. et al. Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell Environ. 37, 1598–1613 (2014).
Allen, C. D., Breshears, D. D. & McDowell, N. G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 129 (2015).
Carnicer, J. et al. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl Acad. Sci. USA 108, 1474–1478 (2011).
Ciais, P. et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533 (2005).
Lewis, S. L., Brando, P. M., Phillips, O. L., van der Heijden, G. M. F. & Nepstad, D. The 2010 Amazon drought. Science 331, 554 (2011).
Asner, G. P. et al. Progressive forest canopy water loss during the 2012–2015 California drought. Proc. Natl Acad. Sci. USA 113, E249–E255 (2016).
Moore, G. W. et al. Tree mortality from an exceptional drought spanning mesic to semiarid ecoregions. Ecol. Appl. 26, 602–611 (2016).
USDA Forest Service Pacific Southwest Region. Aerial Detection Surveys Report: Summary for May 15–19 Report No. fseprd506698 (USDA Forest Service, 2016).
Allen, C. D. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259, 660–684 (2010). This study summarizes forest mortality events associated with drought and heat over the last four decades.
Duke, N. C. et al. Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: a severe ecosystem response, coincidental with an unusually extreme weather event. Mar. Freshw. Res. 68, 1816–1829 (2017).
Phillips, O. L. et al. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009).
da Costa, A. C. L. et al. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytol. 187, 579–591 (2010).
Lindenmayer, D. B. & Laurance, W. F. The ecology, distribution, conservation and management of large old trees. Biol. Rev. Camb. Philos. Soc. 92, 1434–1458 (2016).
Slatyer, R. O. Plant–Water relationships (Academic, New York, 1967).
Debenedetti, P. G. Metastable Liquids: Concepts and Principles (Princeton Univ. Press, Princeton, 1996).
Rodriguez-Dominguez, C. M., Carins Murphy, M. R., Lucani, C. & Brodribb, T. J. Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytol. 218, 1025–1035 (2018).
Scholander, P. F., Hammel, H. T., Bradstreet, E. D. & Hemmingsen, E. A. Sap pressure in vascular plants. Science 148, 339–346 (1965).
Hochberg, U. et al. Stomatal closure, basal leaf embolism and shedding protect the hydraulic integrity of grape stems. Plant Physiol. (2017).
Martin-StPaul, N., Delzon, S. & Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 20, 1437–1447 (2017). The absolute limit at which stomata must close to avoid mortality under drought is described.
Li, X. et al. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ. 41, 646–660 (2018).
Leigh, A., Sevanto, S., Close, J. D. & Nicotra, A. B. The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions? Plant Cell Environ. 40, 237–248 (2017).
Powles, S. B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35, 15–44 (1984).
Mitchell, P. J. et al. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 197, 862–872 (2013).
Sevanto, S., McDowell, N. G., Dickman, L. T., Pangle, R. & Pockman, W. T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 37, 153–161 (2014).
Dietze, M. C. & Matthes, J. H. A general ecophysiological framework for modelling the impact of pests and pathogens on forest ecosystems. Ecol. Lett. 17, 1418–1426 (2014).
Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 47, 1813–1832 (1996).
Oren, R. & Pataki, D. E. Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia 127, 549–559 (2001).
Zhang, Y.-J., Rockwell, F. E., Graham, A. C., Alexander, T. & Holbrook, N. M. Reversible leaf xylem collapse: a potential “circuit breaker” against cavitation. Plant Physiol. 172, 2261–2274 (2016).
McElrone, A. J. et al. Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves. Plant Cell Environ. 30, 1411–1421 (2007).
Sack, L. & Holbrook, N. M. Leaf hydraulics. Annu. Rev. Plant Biol. 57, 361–381 (2006).
Cuneo, I. F., Knipfer, T., Brodersen, C. R. & McElrone, A. J. Mechanical failure of fine root cortical cells initiates plant hydraulic decline during drought. Plant Physiol. 172, 1669–1678 (2016).
Borchert, R. & Pockman, W. T. Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Tree Physiol. 25, 457–466 (2005).
Choat, B., Brodersen, C. R. & McElrone, A. J. Synchrotron X-ray microtomography of xylem embolism in Sequoia sempervirens saplings during cycles of drought and recovery. New Phytol. 205, 1095–1105 (2015).
Brodribb, T. J. et al. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol. 209, 1403–1409 (2016).
Tyree, M. T., Cochard, H., Cruiziat, P., Sinclair, B. & Ameglio, T. Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant Cell Environ. 16, 879–882 (1993).
Rood, S. B., Patiño, S., Coombs, K. & Tyree, M. T. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14, 248–257 (2000).
Choat, B. et al. Noninvasive measurement of vulnerability to drought-induced embolism by X-ray microtomography. Plant Physiol. 170, 273–282 (2016).
Larter, M. et al. Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol. 215, 97–112 (2017).
Pittermann, J. The evolution of water transport in plants: an integrated approach. Geobiology 8, 112–139 (2010).
Blackman, C. J. et al. Toward an index of desiccation time to tree mortality under drought. Plant Cell Environ. 39, 2342–2345 (2016). A process-based approach is used to model desiccation time to mortality in trees under drought.
Bartlett, M. K., Klein, T., Jansen, S., Choat, B. & Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl Acad. Sci. USA 113, 13098–13103 (2016).
Choat, B. et al. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755 (2012). Global synthesis demonstrating a convergence in tree hydraulic safety margins across forest biomes.
Maherali, H., Pockman, W. T. & Jackson, R. B. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85, 2184–2199 (2004).
Lens, F. et al. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 190, 709–723 (2011).
Pittermann, J. et al. The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: the evolution of pit membrane form and function. Plant Physiol. 153, 1919–1931 (2010).
Blackman, C. J., Brodribb, T. J. & Jordan, G. J. Leaf hydraulic vulnerability influences species’ bioclimatic limits in a diverse group of woody angiosperms. Oecologia 168, 1–10 (2012).
Mencuccini, M., Minunno, F., Salmon, Y., Martínez-Vilalta, J. & Hölttä, T. Coordination of physiological traits involved in drought-induced mortality of woody plants. New Phytol. 208, 396–409 (2015).
Reich, P. B. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Anderegg, W. R. et al. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl Acad. Sci. USA 113, 5024–5029 (2016).
Rowland, L. et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528, 119–122 (2015).
Lamy, J.-B. et al. Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytol. 201, 874–886 (2014).
Schuldt, B. et al. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 210, 443–458 (2016).
Mencuccini, M. & Grace, J. Climate influences the leaf area/sapwood area ratio in Scots pine. Tree Physiol. 15, 1–10 (1995).
Magnani, F., Mencuccini, M. & Grace, J. Age-related decline in stand productivity: the role of structural acclimation under hydraulic constraints. Plant Cell Environ. 23, 251–263 (2000).
Maherali, H. & DeLucia, E. H. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiol. 20, 859–867 (2000).
Martínez-Vilalta, J. et al. Hydraulic adjustment of Scots pine across Europe. New Phytol. 184, 353–364 (2009). Comprehensive study of intra-specific variation in hydraulic traits across a broad climatic gradient.
Wortemann, R. et al. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol. 31, 1175–1182 (2011).
Hogg, E. H., Brandt, J. P. & Kochtubajda, B. Growth and dieback of aspen forests in northwestern Alberta, Canada, in relation to climate and insects. Can. J. For. Res. 32, 823–832 (2002).
Sperry, J. S. & Love, D. M. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 207, 14–27 (2015).
Delzon, S. & Cochard, H. Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol. 203, 355–358 (2014).
Anderegg, W. R. L. et al. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 8, 367–371 (2015).
McDowell, N. G. et al. Multi-scale predictions of massive conifer mortality due to chronic temperature rise. Nat. Clim. Change 6, 295–300 (2016).
Brodribb, T. J., Bowman, D. J. M. S., Nichols, S., Delzon, S. & Burlett, R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 188, 533–542 (2010).
Meinzer, F. C., Johnson, D. M., Lachenbruch, B., McCulloh, K. A. & Woodruff, D. R. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 23, 922–930 (2009).
McDowell, N. G. et al. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytol. 200, 304–321 (2013).
Gustafson, E. J. & Sturtevant, B. R. Modeling forest mortality caused by drought stress: implications for climate change. Ecosystems 16, 60–74 (2013).
Mitchell, P. J. et al. An ecoclimatic framework for evaluating the resilience of vegetation to water deficit. Glob. Chang. Biol. 22, 1677–1689 (2016).
O’Sullivan, O. S. et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Biol. 23, 209–223 (2017).
Adams, H. D. et al. Empirical and process-based approaches to climate-induced forest mortality models. Front. Plant Sci. 4, 438 (2013).
Christoffersen, B. O. et al. Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS v.1-Hydro). Geosci. Model Dev. 9, 4227–4255 (2016).
Xu, X., Medvigy, D., Powers, J. S., Becknell, J. M. & Guan, K. Diversity in plant hydraulic traits explains seasonal and inter-annual variations of vegetation dynamics in seasonally dry tropical forests. New Phytol. 212, 80–95 (2016).
Davi, H. & Cailleret, M. Assessing drought-driven mortality trees with physiological process-based models. Agric. For. Meteorol. 232, 279–290 (2017).
Bartlett, M. K., Scoffoni, C. & Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol. Lett. 15, 393–405 (2012). Data synthesis that links the point at which leaf turgor is lost to drought tolerance in plants.
Limousin, J.-M., Longepierre, D., Huc, R. & Rambal, S. Change in hydraulic traits of Mediterranean Quercus ilex subjected to long-term throughfall exclusion. Tree Physiol. 30, 1026–1036 (2010).
Vilagrosa, A., Bellot, J., Vallejo, V. R. & Gil-Pelegrín, E. Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J. Exp. Bot. 54, 2015–2024 (2003).
Dahlin, K. M., Ponte, D. D., Setlock, E. & Nagelkirk, R. Global patterns of drought deciduous phenology in semi-arid and savanna-type ecosystems. Ecography 40, 314–323 (2016).
De Kauwe, M. G. et al. Do land surface models need to include differential plant species responses to drought? Examining model predictions across a mesic–xeric gradient in Europe. Biogeosciences 12, 7503–7518 (2015).
Aguadé, D., Poyatos, R., Rosas, T. & Martínez-Vilalta, J. Comparative drought responses of Quercus ilex L. and Pinus sylvestris L. in a montane forest undergoing a vegetation shift. Forests 6, 2505 (2015).
Donovan, L., Linton, M. & Richards, J. Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129, 328–335 (2001).
Nobel, P. S. & Cui, M. Hydraulic conductances of the soil, the root–soil air gap, and the root: changes for desert succulents in drying soil. J. Exp. Bot. 43, 319–326 (1992).
Eller, C. B., Lima, A. L. & Oliveira, R. S. Cloud forest trees with higher foliar water uptake capacity and anisohydric behavior are more vulnerable to drought and climate change. New Phytol. 211, 489–501 (2016).
Sinclair, T. R. Model analysis of plant traits leading to prolonged crop survival during severe drought. Field Crops Res. 68, 211–217 (2000).
Manzoni, S., Katul, G. & Porporato, A. A dynamical system perspective on plant hydraulic failure. Wat. Resour. Res. 50, 5170–5183 (2014).
Gentine, P., Guérin, M., Uriarte, M., McDowell, N. G. & Pockman, W. T. An allometry-based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrology 9, 529–546 (2016).
Waring, R. H. Characteristics of trees predisposed to die. Bioscience 37, 569–574 (1987).
Bréda, N., Huc, R., Granier, A. & Dreyer, E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 63, 625–644 (2006).
De Micco, V., Balzano, A., Wheeler, E. A. & Baas, P. Tyloses and gums: a review of structure, function and occurrence of vessel occlusions. IAWA J. 37, 186–205 (2016).
Zeppel, M. J. B. et al. Drought and resprouting plants. New Phytol. 206, 583–589 (2015).
Bond, W. J. & Midgley, J. J. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol. 16, 45–51 (2001).
Brodersen, C. R. & McElrone, A. J. Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front. Plant Sci. 4, 108 (2013).
Zwieniecki, M. A. & Holbrook, N. M. Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci. 14, 530–534 (2009).
Nardini, A., Savi, T., Trifilò, P. & Lo Gullo, M. A. in Progress in Botany Vol. 79(eds Cánovas, F. et al.) 197–231 (Springer, Cham, 2017).
Cobb, A. R., Choat, B. & Holbrook, N. M. Dynamics of freeze–thaw embolism in Smilax rotundifolia (Smilacaceae). Am. J. Bot. 94, 640–649 (2007).
Cochard, H., Lemoine, D., Améglio, T. & Granier, A. Mechanisms of xylem recovery from winter embolism in Fagus sylvatica. Tree Physiol. 21, 27–33 (2001).
Sperry, J. S., Holbrook, N. M., Zimmermann, M. H. & Tyree, M. T. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 83, 414–417 (1987).
Kaufmann, I. et al. Functional repair of embolized vessels in maize roots after temporal drought stress, as demonstrated by magnetic resonance imaging. New Phytol. 184, 245–256 (2009).
McCully, M. E., Huang, C. X. & Ling, L. E. C. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 138, 327–342 (1998).
Taneda, H. & Sperry, J. S. A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol. 28, 1641–1651 (2008).
Zwieniecki, M. A. & Holbrook, N. M. Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.). Plant Cell Environ. 21, 1173–1180 (1998).
Brodersen, C. R., McElrone, A. J., Choat, B., Matthews, M. A. & Shackel, K. A. The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol. 154, 1088–1095 (2010). First study to utilize synchrotron-based imaging methods for non-destructive visualization of xylem function.
Charrier, G. et al. Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiol. 172, 1657–1668 (2016).
Clearwater, M. J. & Clark, C. J. In vivo magnetic resonance imaging of xylem vessel contents in woody lianas. Plant Cell Environ. 26, 1205–1214 (2003).
Knipfer, T., Brodersen, C. R., Zedan, A., Kluepfel, D. A. & McElrone, A. J. Patterns of drought-induced embolism formation and spread in living walnut saplings visualized using X-ray microtomography. Tree Physiol. 35, 744–755 (2015).
Skelton, R. P., Brodribb, T. J., McAdam, S. A. M. & Mitchell, P. J. Gas exchange recovery following natural drought is rapid unless limited by loss of leaf hydraulic conductance: evidence from an evergreen woodland. New Phytol. 215, 1399–1412 (2017).
Fukuda, H. Xylogenesis: initiation, progression, and cell death. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 299–325 (1996).
Davies, W., Metcalfe, J., Lodge, T. & da Costa, A. R. Plant growth substances and the regulation of growth under drought. Funct. Plant Biol. 13, 105–125 (1986).
Liang, E., Balducci, L., Ren, P. & Rossi, S. in Secondary Xylem Biology: Origins, Functions, and Applications (eds Kim, Y.S. et al.) 45–58 (Academic, Cambridge, 2016).
Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon? – How trees may cope with more frequent climate change-type drought events. Glob. Chang. Biol. 17, 642–655 (2011).
Brunner, I., Herzog, C., Dawes, M. A., Arend, M. & Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 6, 547 (2015).
Kozlowski, T. & Pallardy, S. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 68, 270–334 (2002).
Pagay, V. et al. A microtensiometer capable of measuring water potentials below −10 MPa. Lab Chip 14, 2806–2817 (2014).
Luo, Z. et al. Responses of plant water use to a severe summer drought for two subtropical tree species in the central southern China. J. Hydrol. 8, 1–9 (2016).
Cochard, H. et al. Methods for measuring plant vulnerability to cavitation: a critical review. J. Exp. Bot. 64, 4779–4791 (2013).
Poyatos, R. et al. SAPFLUXNET: towards a global database of sap flow measurements. Tree Physiol. 36, 1449–1455 (2016).
McDowell, N. G. et al. Global satellite monitoring of climate-induced vegetation disturbances. Trends Plant Sci. 20, 114–123 (2015).
Acknowledgements
We thank S. Stuart, H. Cochard and M. Holbrook for insightful comments and discussion during the preparation of the Review. Micro-computed tomography images included in Fig. 2 were collected during beam-time allocations at the Imaging and Medical beam line (Australian Synchrotron) and TOMCAT beam line (Swiss Light Source). B.C., T.J.B. and B.E.M. acknowledge support from the Australian Research Council (FT130101115; LP140100232; DP170100761). R.L. was supported by a Marie Curie Fellowship (FP7PEOPLE-2013-IOF-624473).
Reviewer information
Nature thanks B. Engelbrecht, N. G. McDowell and M. Mencuccini for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and planning of the manuscript. B.C., T.J.B. and B.E.M. developed the initial outline and synopsis of the Review. B.C. was responsible for the coordination of the writing of the manuscript. B.C. and C.R.B. prepared figures and the table.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
This file contains a list of plant traits (physiological and morphological) that determine rate of decline in plant water potential during drought and thresholds of hydraulic failure.
Rights and permissions
About this article
Cite this article
Choat, B., Brodribb, T.J., Brodersen, C.R. et al. Triggers of tree mortality under drought. Nature 558, 531–539 (2018). https://doi.org/10.1038/s41586-018-0240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0240-x
This article is cited by
-
Inter-provenance variability and phenotypic plasticity of wood and leaf traits related to hydraulic safety and efficiency in seven European beech (Fagus sylvatica L.) provenances differing in yield
Annals of Forest Science (2024)
-
The fluidic memristor as a collective phenomenon in elastohydrodynamic networks
Nature Communications (2024)
-
The xylem functional traits of eight subtropical tree species is closely related to the intervessel pits ultrastructure
Trees (2024)
-
Young temperate tree species show different fine root acclimation capacity to growing season water availability
Plant and Soil (2024)
-
High sensitivity of hop plants (Humulus lupulus L.) to limited soil water availability: the role of stomata regulation and xylem vulnerability to embolism
Irrigation Science (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.