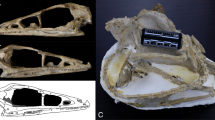
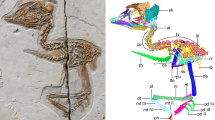
Abstract
The skull of living birds is greatly modified from the condition found in their dinosaurian antecedents. Bird skulls have an enlarged, toothless premaxillary beak and an intricate kinetic system that includes a mobile palate and jaw suspensorium. The expanded avian neurocranium protects an enlarged brain and is flanked by reduced jaw adductor muscles. However, the order of appearance of these features and the nature of their earliest manifestations remain unknown. The Late Cretaceous toothed bird Ichthyornis dispar sits in a pivotal phylogenetic position outside living groups: it is close to the extant avian radiation but retains numerous ancestral characters1,2,3. Although its evolutionary importance continues to be affirmed3,4,5,6,7,8, no substantial new cranial material of I. dispar has been described beyond incomplete remains recovered in the 1870s. Jurassic and Cretaceous Lagerstätten have yielded important avialan fossils, but their skulls are typically crushed and distorted9. Here we report four three-dimensionally preserved specimens of I. dispar—including an unusually complete skull—as well as two previously overlooked elements from the Yale Peabody Museum holotype, YPM 1450. We used these specimens to generate a nearly complete three-dimensional reconstruction of the I. dispar skull using high-resolution computed tomography. Our study reveals that I. dispar had a transitional beak—small, lacking a palatal shelf and restricted to the tips of the jaws—coupled with a kinetic system similar to that of living birds. The feeding apparatus of extant birds therefore evolved earlier than previously thought and its components were functionally and developmentally coordinated. The brain was relatively modern, but the temporal region was unexpectedly dinosaurian: it retained a large adductor chamber bounded dorsally by substantial bony remnants of the ancestral reptilian upper temporal fenestra. This combination of features documents that important attributes of the avian brain and palate evolved before the reduction of jaw musculature and the full transformation of the beak.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huang, J. et al. A new ornithurine from the Early Cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds. PeerJ 4, e1765 (2016).
Marsh, O. C. Notice of a new and remarkable fossil bird. Am. J. Sci. 4 (third series), 344 (1872).
Clarke, J. A. Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bull. Am. Mus. Nat. Hist. 286, 1–179 (2004).
Olson, S. L. Ichthyornis in the Cretaceous of Alabama. Wilson Bull. 87, 103–105 (1975).
Lucas, S. G. & Sullivan, R. M. Ichthyornis in the Late Cretaceous Mancos Shale (Juana Lopez Member), northwestern New Mexico. J. Paleontol. 56, 545–547 (1982).
Parris, D. & Echols, J. The fossil bird Ichthyornis in the Cretaceous of Texas. Tex. J. Sci. 44, 201–212 (1992).
Shimada, T. R. & Wilson, L. E. A new specimen of the Late Cretaceous bird, cf. Ichthyornis sp., from the Cenomanian of central Kansas, with comments on the size distribution of Ichthyornis in North America. Trans. Kans. Acad. Sci. 119, 231–237 (2016).
Gingerich, P. D. A new partial mandible of Ichthyornis. Condor 74, 471–473 (1972).
Chiappe, L. M. & Qingjin, M. Birds of Stone: Chinese Avian Fossils from the Age of Dinosaurs (Johns Hopkins Univ. Press, Baltimore, 2016).
Felice, R. N. & Goswami, A. Developmental origins of mosaic evolution in the avian cranium. Proc. Natl Acad. Sci. USA 115, 555–560 (2018).
Mayr, G. Avian Evolution: the Fossil Record of Birds and its Paleobiological Significance (John Wiley & Sons, Oxford, 2016).
Gauthier, J. in The Origin of Birds and the Evolution of Flight (Memoirs of the California Academy of Sciences 8) (ed. Padian, K.) 1–55 (California Academy of Sciences, San Francisco, 1986).
Zhou, Z. & Martin, L. D. Distribution of the predentary bone in Mesozoic ornithurine birds. J. Syst. Palaeontology 9, 25–31 (2011).
Bhullar, B.-A. S. et al. How to make a bird skull: major transitions in the evolution of the avian cranium, paedomorphosis, and the beak as a surrogate hand. Integr. Comp. Biol. 56, 389–403 (2016).
O’Connor, J. The trophic habits of early birds. Palaeogeogr. Palaeoclimatol. Palaeoecol. https://doi.org/10.1016/j.palaeo.2018.03.006 (2018).
Zheng, X., O’Connor, J. K., Wang, X., Wang, Y. & Zhou, Z. Reinterpretation of a previously described Jehol bird clarifies early trophic evolution in the Ornithuromorpha. Proc. R. Soc. B 285, 20172494 (2018).
Hieronymus, T. L. & Witmer, L. M. Homology and evolution of avian compound rhamphothecae. Auk 127, 590–604 (2010).
Baumel, J. J. et al. Handbook of Avian Anatomy: Nomina Anatomica Avium (Publications of the Nuttall Ornithological Club, No. 23) 2nd edn (Harvard Univ. Nuttal Ornithological, Cambridge, 1993).
Elzanowski, A. New observations on the skull of Hesperornis with reconstructions of the bony palate and otic region. Postilla 207, 1–20 (1991).
Bock, W. J. Kinetics of the avian skull. J. Morphol. 114, 1–42 (1964).
Homberger, D. G. in The Biology of the Avian Respiratory System (ed. Maina, J. N.) 27–97 (Springer, Cham, 2017).
Martin, L. D. & Stewart, J. D. Teeth in Ichthyornis (Class: Aves). Science 195, 1331–1332 (1977).
Dumont, M. et al. Synchrotron imaging of dentition provides insights into the biology of Hesperornis and Ichthyornis, the “last” toothed birds. BMC Evol. Biol. 16, 178 (2016).
Jollie, M. T. The head skeleton of the chicken and remarks on the anatomy of this region in other birds. J. Morphol. 100, 389–436 (1957).
Zusi, R. L. in The Skull, Volume 2: Patterns of Structural and Systematic Diversity (eds. Hanken, J. & Hall, B. K.) Ch. 8, 391–437 (Univ. of Chicago Press, Chicago, 1993).
Chiappe, L. M., Norell, M. & Clark, J. A new skull of Gobipteryx minuta (Aves: Enantiornithes) from the Cretaceous of the Gobi Desert. Am. Mus. Novit. 3346, 1–15 (2001).
Bühler, P., Martin, L. D. & Witmer, L. M. Cranial kinesis in the Late Cretaceous birds Hesperornis and Parahesperornis. Auk 105, 111–122 (1988).
Zusi, R. L. A functional and evolutionary analysis of rhynchokinesis in birds. Smithson. Contrib. Zool. 395, 1–40 (1984).
Gussekloo, S. W. & Bout, R. G. Cranial kinesis in palaeognathous birds. J. Exp. Biol. 208, 3409–3419 (2005).
Rauhut, O. W. M. New observations on the skull of Archaeopteryx. Palaontol Z. 88, 211–221 (2014).
Mayr, G., De Pietri, V. L., Scofield, R. P. & Worthy, T. H. On the taxonomic composition and phylogenetic affinities of the recently proposed clade Vegaviidae Agnolín et al., 2017‒neornithine birds from the Upper Cretaceous of the Southern Hemisphere. Cretac. Res. 86, 178–185 (2018).
Walsh, S. A., Milner, A. C. & Bourdon, E. A reappraisal of Cerebavis cenomanica (Aves, Ornithurae), from Melovatka, Russia. J. Anat. 229, 215–227 (2016).
Alonso, P. D., Milner, A. C., Ketcham, R. A., Cookson, M. J. & Rowe, T. B. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666–669 (2004).
Balanoff, A. M., Bever, G. S., Rowe, T. B. & Norell, M. A. Evolutionary origins of the avian brain. Nature 501, 93–96 (2013).
Elzanowski, A. & Galton, P. M. Braincase of Enaliornis, an Early Cretaceous bird from England. J. Vertebr. Paleontol. 11, 90–107 (1991).
Elzanowski, A. & Mayr, G. Multiple origins of secondary temporal fenestrae and orbitozygomatic junctions in birds. J. Zoolog. Syst. Evol. Res. https://doi.org/10.1111/jzs.12196 (2017).
Müller, H. J. Die morphologie und entwicklung des craniums von Rhea americana Linné. II. Viszeralskelett, mittelohr und osteocranium. Z. Wiss. Zool. 168, 35–118 (1963).
Cracraft, J. The origin and early diversification of birds. Paleobiology 12, 383–399 (1986).
Holliday, C. M. & Witmer, L. M. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J. Morphol. 268, 457–484 (2007).
Bhullar, B. A. S. et al. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution 69, 1665–1677 (2015).
Acknowledgements
We thank M. Colbert, J. Maisano and the staff of the UTCT facility at The University of Texas at Austin, as well as G. Lin at the Harvard Center for Nanoscale Systems and A. Pritchard at Yale for CT scanning assistance. K. Zyskowski and G. Watkins-Colwell in the Division of Vertebrate Zoology at YPM assisted with extant comparative material. M. Fox, C. Norris and D. Brinkman facilitated the examination and scanning of YPM fossil material. This research was supported by Yale University, the Yale Peabody Museum of Natural History, the University of Bath, National Science Foundation Doctoral Dissertation Improvement Grant DEB 1500798, the Alexander Wetmore Memorial Research Award (American Ornithologists’ Union), a Yale Institute for Biospheric Studies Dissertation Improvement Grant, the Stephen J. Gould Award (Paleontological Society), an Evolving Earth Foundation Research Grant and a Frank M. Chapman Ornithological Research Grant (American Museum of Natural History).
Reviewer information
Nature thanks Z. Zhou and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.J.F. and B.-A.S.B. conceived and directed the study and arranged logistics of specimen preparation and CT scanning. K.S. discovered the FHSM specimen and donated it to the museum. D.J.F., M.H. and B.-A.S.B. performed CT scans and processed CT data and M.H. assembled the skull reconstruction. M.H., D.J.F. and B.-A.S.B. scored characters and performed phylogenetic analyses. B.-A.S.B. and M.H. planned the main-text figures. M.H. and D.J.F. prepared the figures. D.J.F. wrote the supplementary anatomical descriptions and rendered the Supplementary Videos. D.B., L.E.W., K.S., D.E. and J.A.E. collected and prepared specimens for study, analysed morphology and edited the paper. B.-A.S.B., D.J.F. and M.H. wrote the paper. B.-A.S.B. and D.J.F. acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Full 3D reconstruction of the skull of I. dispar in high resolution.
This is the same reconstruction as shown in Fig. 1, reproduced at a higher resolution to show details.
Extended Data Fig. 2 Reconstruction of the skull of I. dispar.
Material described in this paper is indicated in gold and previously described regions are indicated in grey. All elements are scaled to the size of the FHSM VP-18702 specimen.
Extended Data Fig. 3 Reconstruction of the skull of I. dispar indicating the material represented by every known Ichthyornis specimen.
All elements are scaled to the size of the FHSM VP-18702 specimen. Specimen numbers in bold are those used in the reconstruction. Numbers in italics indicate preservation of the same element in additional specimens.
Extended Data Fig. 5
Skull and jaw elements of I. dispar specimen BHI 6421.
Extended Data Fig. 6
Skull and jaw elements of I. dispar specimen FHSM VP-18702.
Extended Data Fig. 7
Skull and jaw elements of I. dispar specimen KUVP 119673.
Extended Data Fig. 8
Skull and jaw elements of I. dispar specimen ALMNH 3316.
Extended Data Fig. 9
Skull and jaw elements of I. dispar holotype YPM 1450 showing the nasal and lacrimal elements that have not previously been reported.
Extended Data Fig. 10
Skull and jaw elements of I. dispar specimens YPM 1728, YPM 1459, YPM 1775 and YPM 1749.
Supplementary information
Supplementary Information
This file contains supplementary text 1-9; which contains supplementary tree legends for 1-17, supplementary video legends 1-7, supplementary table 1 and supplementary figure 1
Supplementary Data
This file contains Supplementary Trees 1-17. (Tree legends can be found in the Supplementary Information)
41586_2018_53_MOESM7_ESM.mov
Video 4: YPM 1450, nasal and lacrimal. Partial nasal and lacrimal are attached as they were discovered in the reanalysis of YPM 1450
Rights and permissions
About this article
Cite this article
Field, D.J., Hanson, M., Burnham, D. et al. Complete Ichthyornis skull illuminates mosaic assembly of the avian head. Nature 557, 96–100 (2018). https://doi.org/10.1038/s41586-018-0053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0053-y
This article is cited by
-
Decoupling the skull and skeleton in a Cretaceous bird with unique appendicular morphologies
Nature Ecology & Evolution (2023)
-
Cretaceous ornithurine supports a neognathous crown bird ancestor
Nature (2022)
-
A non-avian dinosaur with a streamlined body exhibits potential adaptations for swimming
Communications Biology (2022)
-
Fossil find suggests ancestral bird beak was mobile
Nature (2022)
-
Cretaceous bird with dinosaur skull sheds light on avian cranial evolution
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.