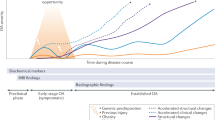
Abstract
Knee osteoarthritis (OA) is a heterogeneous disease associated with substantial effects on quality of life, and its clinical management is difficult. Among the several available guidelines for the management of knee OA, those from OARSI and ESCEO were updated in 2019. Here, we examine the similarities and differences between these two guidelines and provide a narrative to help guide health-care providers through the complexities of non-surgical management of knee OA. OARSI and ESCEO both recommend education, structured exercise and weight loss as core treatments, topical NSAIDs as first-line treatments and oral NSAIDs and intra-articular injections for persistent pain. Low-dose, short-term acetaminophen, pharmaceutical grade glucosamine and chondroitin sulfate are recommended by ESCEO whereas OARSI strongly recommends against their use (including all glucosamine and chondroitin formulations). Despite this difference, the two guidelines are consistent in the majority of their recommendations and provide useful treatment recommendations for individuals with OA and health-care providers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Safiri, S. et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 79, 819–828 (2020).
Disease, G. B. D. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018).
Culliford, D. et al. Future projections of total hip and knee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. Osteoarthritis Cartilage 23, 594–600 (2015).
McAlindon, T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22, 363–388 (2014).
Jordan, K. M. et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 62, 1145–1155 (2003).
Bruyere, O. et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 44, 253–263 (2014).
Hochberg, M. C. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 64, 465–474 (2012).
Jevsevar, D. S. et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J. Bone Joint Surg. Am. 95, 1885–1886 (2013).
Kolasinski, S. L. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72, 220–233 (2020).
Bijlsma, J. W. J., Berenbaum, F. & Lafeber, F. P. J. G. Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 (2011).
Buttgereit, F., Burmester, G.-R. & Bijlsma, J. W. J. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open 1, e000027 (2015).
Healey, E. L. et al. Uptake of the NICE osteoarthritis guidelines in primary care: a survey of older adults with joint pain. BMC Musculoskelet. Disord. 19, 295 (2018).
Carlson, V. R. et al. Compliance with the AAOS guidelines for treatment of osteoarthritis of the knee: a survey of the American Association of Hip and Knee Surgeons. J. Am. Acad. Orthop. Surg. 26, 103–107 (2018).
Basedow, M., Williams, H., Shanahan, E. M., Runciman, W. B. & Esterman, A. Australian GP management of osteoarthritis following the release of the RACGP guideline for the non-surgical management of hip and knee osteoarthritis. BMC Res. Notes 8, 536 (2015).
Nelson, A. E., Allen, K. D., Golightly, Y. M., Goode, A. P. & Jordan, J. M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin. Arthritis Rheum. 43, 701–712 (2014).
Bruyere, O. et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 49, 337–350 (2019).
Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 27, 1578–1589 (2019).
Price, A. J. et al. Knee replacement. Lancet 392, 1672–1682 (2018).
Dieppe, P., Lim, K. & Lohmander, S. Who should have knee joint replacement surgery for osteoarthritis? Int. J. Rheum. Dis. 14, 175–180 (2011).
Beswick, A. D., Wylde, V., Gooberman-Hill, R., Blom, A. & Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2, e000435 (2012).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 15, 981–1000 (2007).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16, 137–162 (2008).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18, 476–499 (2010).
Lutzner, J., Kasten, P., Gunther, K.-P. & Kirschner, S. Surgical options for patients with osteoarthritis of the knee. Nat. Rev. Rheumatol. 5, 309–316 (2009).
Beard, D. J. et al. The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5-year outcomes of a randomised controlled trial. Lancet 394, 746–756 (2019).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Atkins, D. et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 4, 38 (2004).
Atkins, D. et al. Grading quality of evidence and strength of recommendations. BMJ 328, 1490 (2004).
Kavanagh, B. P. The GRADE system for rating clinical guidelines. PLoS Med. 6, e1000094 (2009).
Huang, X., Lin, J. & Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 359–363 (2006).
Cheng, G. Y. T. A study of clinical questions posed by hospital clinicians. J. Med. Libr. Assoc. 92, 445–458 (2004).
Sostres, C., Gargallo, C. J., Arroyo, M. T. & Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 24, 121–132 (2010).
Bhala, N., Emberson, J., Patrono, C., Baigent, C. & Collaborators, C. N. T. Coxibs and traditional NSAIDs for pain relief – Authors’ reply. Lancet 383, 122 (2014).
2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694 (2019).
Kahan, A., Uebelhart, D., De Vathaire, F., Delmas, P. D. & Reginster, J.-Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 60, 524–533 (2009).
Zegels, B., Crozes, P., Uebelhart, D., Bruyere, O. & Reginster, J. Y. Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis. Results of a randomized double blind placebo controlled study. Osteoarthritis Cartilage 21, 22–27 (2013).
Schneider, H., Maheu, E. & Cucherat, M. Symptom-modifying effect of chondroitin sulfate in knee osteoarthritis: a meta-analysis of randomized placebo-controlled trials performed with Structum®. Open Rheumatol. J. 6, 183–189 (2012).
Reginster, J.-Y., Dudler, J., Blicharski, T. & Pavelka, K. Pharmaceutical-grade chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann. Rheum. Dis. 76, 1537–1543 (2017).
Acknowledgements
Authors’ statement: the diverse views and opinions expressed in this article represent the outcomes of a joint working group, consisting of current members of the Osteoarthritis Research Society International (OARSI) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), which examined the similarities and differences between the 2019 OARSI and ESCEO guidelines for the management of knee OA. The working group was entirely funded by ESCEO. To support its educational and scientific activities, ESCEO receives unrestricted educational grants from non-governmental organizations, not-for-profit organizations, and non-commercial and corporate partners. The choice of topics, participants, content and agenda of the working group, as well as the writing, editing, submission and reviewing of the manuscript are the sole responsibility of ESCEO, without any influence from third parties. ESCEO is supported by the Chair for Biomarkers of Chronic Diseases and the International Scientific Partnership Program (ISPP#0111) at King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
T.A.P., N.K.A., R.R.B. and O.B. researched data for the article. T.A.P. and N.K.A. wrote the manuscript. All authors made a substantial contribution to discussion of content and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.K.A. declares that he has received grants and personal fees from Merck, and personal fees from Flexion, Regeneron, Pfizer, and Eli Lilly. R.R.B. is supported by the NIH National Center for Complementary and Integrative Health (K23AT009374). O.B. declares that he has received grants from Biophytis, IBSA, MEDA, Servier, SMB and Theramex. C.C. declares that he has received lecture fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestlé, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB, outside the submitted work. I.K.H. declares that she has received honoraria from Abbvie and a research grant from Pfizer, outside the submitted work. M.C.H. declares that he has acted as a consultant, including attending at Advisory Board meetings, for Bone Therapeutics, Bristol Myers Squibb, Eli Lilly, EMD Serono, Gilead, GlaxoSmithKline, IBSA Institut Biochimique SA, Novartis Pharma AG, Noven Pharmaceuticals, Pfizer, Regenosine, Samumed LLC, Theralogix LLC and Vizuri Health Sciences, and that he has received royalties from Elsevier (for his roles as editor of Rheumatology and Editor-in-Chief of Seminars in Arthritis and Rheumatism) and Wolters Kluwer (UpToDateTM) outside the submitted work. T.E.M. declares that he has acted as a consultant for Samumed, Kolon TissueGene, Pfizer, Sanofi, Regeneron, Noven, Remedium-Bio. A.M. declares that he has acted as a consultant for Abbvie, Aché (Aché Laboratórios Farmacêuticos), Artialis SA, Flexion Therapeutics, Galapagos, Genacol, GSK Consumer Healthcare, IAG, Kolon TissueGene, Pfizer, Pfizer Consumer Healthcare, Servier, Sterifarma, Sanofi and Pacira Biosciences, has received research funding from the European Commission (FP7, IMI, Marie Skłodowska-Curie, ES Struktūrinės Paramos), Versus Arthritis (formerly Arthritis Research UK) and has initiated research contracts with Merck KGaA and Kolon TissueGene, and has received speaker’s fees from Bioiberica SA, the Korean Society for Osteoarthritis and Cartilage Repair, the American College of Rheumatology, the Spanish Society of Rheumatology, the Heilongjiang Rheumatology Association and the Zhujiang Hospital of Southern Medical University. J.-Y.R. declares that he has received grants and personal fees from IBSA-Genevrier, Mylan, and Radius Health, grants from CNIEL, and personal fees from Dairy Research Council and Pierre Fabre, outside of submitted work. T.A.P. declares no conflicts of interest.
Additional information
Peer review information
Nature Reviews Rheumatology thanks E. Maheu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arden, N.K., Perry, T.A., Bannuru, R.R. et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol 17, 59–66 (2021). https://doi.org/10.1038/s41584-020-00523-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00523-9
This article is cited by
-
Development of the intelligent knee osteoarthritis lifestyle app: a person-based approach
BMC Musculoskeletal Disorders (2024)
-
M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis
Journal of Nanobiotechnology (2024)
-
Cartilage-like protein-polysaccharide hybrid hydrogel for enhancing chondrogenic differentiation of bone marrow mesenchymal stem cells
Collagen and Leather (2024)
-
The effects of manual therapy in pain and safety of patients with knee osteoarthritis: a systematic review and meta-analysis
Systematic Reviews (2024)
-
H3K36 methyltransferase NSD1 protects against osteoarthritis through regulating chondrocyte differentiation and cartilage homeostasis
Cell Death & Differentiation (2024)