Abstract
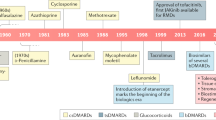
The past century has been characterized by intensive efforts, within both academia and the pharmaceutical industry, to introduce new treatments to individuals with rheumatic autoimmune inflammatory diseases (RAIDs), often by ‘borrowing’ treatments already employed in one RAID or previously used in an entirely different disease, a concept known as drug repurposing. However, despite sharing some clinical manifestations and immune dysregulation, disease pathogenesis and phenotype vary greatly among RAIDs, and limited understanding of their aetiology has made repurposing drugs for RAIDs challenging. Nevertheless, the past century has been characterized by different ‘waves’ of repurposing. Early drug repurposing occurred in academia and was based on serendipitous observations or perceived disease similarity, often driven by the availability and popularity of drug classes. Since the 1990s, most biologic therapies have been developed for one or several RAIDs and then tested among the others, with varying levels of success. The past two decades have seen data-driven repurposing characterized by signature-based approaches that rely on molecular biology and genomics. Additionally, many data-driven strategies employ computational modelling and machine learning to integrate multiple sources of data. Together, these repurposing periods have led to advances in the treatment for many RAIDs.
Key points
-
Repurposing drugs for and among rheumatic autoimmune inflammatory diseases (RAIDs) is difficult because of limitations in knowledge surrounding the pathogenesis of these diseases.
-
Clinical and academic-driven drug repurposing was critical for the early identification of treatments for RAIDs.
-
High costs and increased regulation shifted drug repurposing from primarily academia to pharmaceutical companies.
-
Computational repurposing has the ability to elucidate previously unknown drug mechanisms of action and off-target effects.
-
Genomic and genetic data can identify new pathways and targets for drug repurposing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Elliott, M. J. et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheumatol. 36, 1681–1690 (1993).
Weinblatt, M. E. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340, 253–259 (1999).
Kalunian, K. C. et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheumatol. 63, 3918–3930 (2011).
Mease, P. J. et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheumatol. 50, 2264–2272 (2004).
McInnes, I. B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015).
Vivino, F. B. et al. New treatment guidelines for Sjögren’s disease. Rheum. Dis. Clin. North. Am. 42, 531–551 (2016).
Johr, C. R. & Vivino, F. B. Biologic therapy in the treatment of Sjögren’s syndrome: a clinical perspective. Curr. Treat. Options Rheumatol. 4, 85–98 (2018).
Mahieu, M., Strand, V., Simon, L., Lipsky, P. & Ramsey-Goldman, R. A critical review of clinical trials in systemic lupus erythematosus. Lupus 25, 1122–1140 (2016).
Hruskova, Z. & Tesar, V. Lessons learned from the failure of several recent trials with biologic treatment in systemic lupus erythematosus. Expert Opin. Biol. Ther. 18, 989–996 (2018).
Touma, Z. & Gladman, D. D. Current and future therapies for SLE: obstacles and recommendations for the development of novel treatments. Lupus Sci. Med. 4, e000239 (2017).
Langedijk, J., Mantel-Teeuwisse, A. K., Slijkerman, D. S. & Schutjens, M.-H. D. B. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov. Today 20, 1027–1034 (2015).
Papapetropoulos, A. & Szabo, C. Inventing new therapies without reinventing the wheel: the power of drug repurposing. Br. J. Pharmacol. 175, 165–167 (2018).
Ashburn, T. T. & Thor, K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 (2004).
Nosengo, N. Can you teach old drugs new tricks? Nature 534, 314–316 (2016).
Allison, M. NCATS launches drug repurposing program. Nat. Biotechnol. 30, 571–572 (2012).
Norn, S., Permin, H., Kruse, P. R. & Kruse, E. History of gold-with Danish contribution to tuberculosis and rheumatoid arthritis [article in Danish]. Dan. Medicinhist. Arbog 39, 59–80 (2011).
Møllgaard, H. Some of the principal questions in chemotherapy with special regard to heavy metals. Proc. R. Soc. Med. 20, 787 (1927).
Fraser, T. N. Gold treatment in rheumatoid arthritis. Ann. Rheum. Dis. 4, 71–75 (1945).
Empire Rheumatism Council. Gold therapy in rheumatoid arthritis. A multicentre controlled trial conducted by the Research Subcommittee. Bull. Rheum. Dis. 11, 235–238 (1960).
Research Sub-Committee Of The Empire Rheumatism Council. Gold therapy in rheumatoid arthritis: final report of a multicentre controlled trial. Ann. Rheum. Dis. 20, 315–334 (1961).
Wenger, M. E., Alexander, S., Bland, J. H. & Blechman, W. J. Auranofin versus placebo in the treatment of rheumatoid arthritis. Am. J. Med. 75, 123–127 (1983).
Swift, H. F. & Brown, T. M. P. Pathogenic pleuropneumonia-like microorganisms from acute rheumatic exudates and tissues. Science 89, 271–272 (1939).
Kloppenburg, M., Breedveld, F. C., Terwiel, J. P., Mallee, C. & Dijkmans, B. A. Minocycline in active rheumatoid arthritis. A double-blind, placebo-controlled trial. Arthritis Rheumatol. 37, 629–636 (1994).
Tilley, B. C. et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann. Intern. Med. 122, 81–89 (1995).
Svartz, N. Salazopyrin, a new sulfanilamide preparation. A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparations. Acta Med. Scand. 110, 577–598 (1942).
O’Dell, J. R. et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N. Engl. J. Med. 334, 1287–1291 (1996).
Dougados, M. et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheumatol. 38, 618–627 (1995).
Clegg, D. O., Reda, D. J. & Abdellatif, M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: A Department of Veterans Affairs cooperative study. Arthritis Rheumatol. 42, 2325–2329 (1999).
Nissilä, M. et al. Sulfasalazine in the treatment of ankylosing spondylitis. A twenty-six-week, placebo-controlled clinical trial. Arthritis Rheumatol. 31, 1111–1116 (1988).
Gupta, A. K. et al. Sulfasalazine therapy for psoriatic arthritis: a double blind, placebo controlled trial. J. Rheumatol. 22, 894–898 (1995).
Clegg, D. O. et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheumatol. 39, 2013–2020 (1996).
Schlienger, R. G., Bircher, A. J. & Meier, C. R. Minocycline-induced lupus. Dermatology 200, 223–231 (2000).
Clementz, G. L. & Dolin, B. J. Sulfasalazine-induced lupus erythematosus. Am. J. Med. 84, 535–538 (1988).
Davidson, A. M. & Birt, A. R. Quinine bisulfate as a desensitizing agent in treatment of lupus erythematosus. Arch. Dermatol. Syphilol. 37, 247–253 (1938).
Wallace, D. Antimalarials—the ‘real’ advance in lupus. Lupus 10, 385–387 (2001).
Page, F. Treatment of lupus erythematosus with mepacrine. Lancet 258, 755–758 (1951).
Tye, M. J., White, H., Appel, B. & Ansell, H. B. Lupus erythematosus treated with a combination of quinacrine, hydroxychloroquine and chloroquine. N. Engl. J. Med. 260, 63–66 (1959).
Rainsford, K. D., Parke, A. L., Clifford-Rashotte, M. & Kean, W. F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 23, 231–269 (2015).
Hench, P. S. & Kendall, E. C. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc. Staff Meet. Mayo Clin. 24, 181–197 (1949).
The Nobel Prize. The Nobel Prize in Physiology or Medicine 1950. NobelPrize.org https://www.nobelprize.org/prizes/medicine/1950/summary/ (2019).
Dubois, E. L., Commons, R. R., Starr, P., Stein, C. S. & Morrison, R. Corticotropin and cortisone treatment for systemic lupus erythematosus. J. Am. Med. Assoc. 149, 995–1002 (1952).
Soffer, L. J. Corticotropin and cortisone in acute disseminated lupus erythematosus. J. Am. Med. Assoc. 149, 1002 (2011).
Dordick, J. R. & Gluck, E. J. Preliminary clinical trial with prednisone (meticorten) in systemic lupus erythematosus. AMA Arch. Dermatol. 72, 276–278 (1955).
Bollet, A. J. Treatment of systemic lupus erythematosus with prednisone and prednisolone. J. Am. Med. Assoc. 159, 1501 (2011).
Hart, F. D. Cortisone and ACTH in treatment of ankylosing spondylitis. Br. Med. J. 1, 188–190 (1952).
Walshe, J. M. Penicillamine, a new oral therapy for Wilson’s disease. Am. J. Med. 21, 487–495 (1956).
Jaffe, I. A. Rheumatoid arthritis with arteritis; report of a case treated with penicillamine. Ann. Intern. Med. 61, 556–563 (1964).
Multicentre Trial Group. Controlled trial of d(-)penicillamine in severe rheumatoid arthritis. Lancet 301, 275–280 (1973).
Jaffe, I. A. Comparison of the effect of plasmapheresis and penicillamine on the level of circulating rheumatoid factor. Ann. Rheum. Dis. 22, 71–76 (1963).
Lipsky, P. E. & Ziff, M. Inhibition of human helper T cell function in vitro by D-penicillamine and CuSO4. J. Clin. Invest. 65, 1069–1076 (1980).
Steven, M. M., Morrison, M. & Sturrock, R. D. Penicillamine in ankylosing spondylitis: a double blind placebo controlled trial. J. Rheumatol. 12, 735–737 (1985).
Bernacka, K., Tytman, K. & Sierakowski, S. Clinical application of D-penicillamine in ankylosing spondylitis: a 9-month study. Med. Interne 27, 295–301 (1989).
Price, R. & Gibson, T. D-penicillamine and psoriatic arthropathy. Br. J. Rheumatol. 25, 228 (1986).
Dell’era, L., Boati, E., Nebbia, G. & Corona, F. Wilson’s disease treated with penicillamine and lupus erythematosus: related or distinct entities? Minerva Pediatr. 64, 55–57 (2012).
Lin, H. C. et al. Penicillamine induced lupus-like syndrome: a case report. J. Microbiol. Immunol. Infect. 33, 202–204 (2000).
Iwatani, M. et al. Efficacy profile of bucillamine in rheumatoid arthritis patients in a large observational cohort study, IORRA. Mod. Rheumatol. 16, 376–380 (2006).
Gubner, R., August, S. & Ginsberg, V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am. J. Med. Sci. 221, 176–182 (1951).
Weinblatt, M. E. et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N. Engl. J. Med. 312, 818–822 (1985).
Cuellar, M. L. & Espinoza, L. R. Methotrexate use in psoriasis and psoriatic arthritis. Rheum. Dis. Clin. North. Am. 23, 797–809 (1997).
Braun, J. & Sieper, J. Therapy of ankylosing spondylitis and other spondyloarthritides: established medical treatment, anti-TNF-α therapy and other novel approaches. Arthritis Res. 4, 307 (2002).
Carneiro, J. R. & Sato, E. I. Double blind, randomized, placebo controlled clinical trial of methotrexate in systemic lupus erythematosus. J. Rheumatol. 26, 1275–1279 (1999).
Fortin, P. R. et al. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 59, 1796–1804 (2008).
Drinkard, J. P. et al. Azathioprine and prednisone in the treatment of adults with lupus nephritis. Clinical, histological, and immunological changes with therapy. Medicine 49, 411–432 (1970).
Price, E. J., Rigby, S. P., Clancy, U. & Venables, P. J. A double blind placebo controlled trial of azathioprine in the treatment of primary Sjögren’s syndrome. J. Rheumatol. 25, 896–899 (1998).
Fricke, R. & Petersen, D. Treatment of ankylosing spondylitis with cyclophosphamide and azathioprine [article in German]. Verh. Dtsch. Ges. Rheumatol. 1, 189–195 (1969).
Sadowska-Wróblewska, M., Garwolin´ska, H. & Maczyn´ska-Rusiniak, B. A trial of cyclophosphamide in ankylosing spondylitis with involvement of peripheral joints and high disease activity. Scand. J. Rheumatol. 15, 259–264 (1986).
Ginzler, E. M. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med. 353, 2219–2228 (2005).
Yocum, D. E. et al. Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheumatol. 48, 3328–3337 (2003).
Kawai, S. et al. Comparison of tacrolimus and mizoribine in a randomized, double-blind controlled study in patients with rheumatoid arthritis. J. Rheumatol. 33, 2153–2161 (2006).
Dutta, S. & Ahmad, Y. The efficacy and safety of tacrolimus in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 3, 283–291 (2011).
Furst, D. E. et al. Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with methotrexate: a six-month, double-blind, randomized, dose-ranging study. Arthritis Rheumatol. 46, 2020–2028 (2002).
Takahashi, S. et al. Efficacy and safety of tacrolimus for induction therapy in patients with active lupus nephritis. Mod. Rheumatol. 21, 282–289 (2011).
Miyasaka, N., Kawai, S. & Hashimoto, H. Efficacy and safety of tacrolimus for lupus nephritis: a placebo-controlled double-blind multicenter study. Mod. Rheumatol. 19, 606–615 (2009).
Lai, Z.-W. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391, 1186–1196 (2018).
Tugwell, P. et al. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. N. Engl. J. Med. 333, 137–142 (1995).
Devecı, H. & Kobak, S. The efficacy of topical 0.05% cyclosporine A in patients with dry eye disease associated with Sjögren’s syndrome. Int. Ophthalmol. 34, 1043–1048 (2014).
Sumethkul, K., Kitumnuaypong, T., Angthararak, S. & Pichaiwong, W. Low-dose cyclosporine for active lupus nephritis: a dose titration approach. Clin. Rheumatol. 38, 2151–2159 (2019).
Bartlett, R. R. et al. Leflunomide (HWA 486), a novel immunomodulating compound for the treatment of autoimmune disorders and reactions leading to transplantation rejection. Agents Actions 32, 10–21 (1991).
[No authors listed]. Leflunomide approved for rheumatoid arthritis; other drugs nearing approval. Am. J. Health Syst. Pharm. 55, 2225–2226 (1998).
van Woerkom, J. M. et al. Safety and efficacy of leflunomide in primary Sjögren’s syndrome: a phase II pilot study. Ann. Rheum. Dis. 66, 1026–1032 (2007).
van Denderen, J. C. et al. Double blind, randomised, placebo controlled study of leflunomide in the treatment of active ankylosing spondylitis. Ann. Rheum. Dis. 64, 1761–1764 (2005).
Tam, L.-S., Li, E. K., Wong, C.-K., Lam, C. W. & Szeto, C.-C. Double-blind, randomized, placebo-controlled pilot study of leflunomide in systemic lupus erythematosus. Lupus 13, 601–604 (2004).
Remer, C. F., Weisman, M. H. & Wallace, D. J. Benefits of leflunomide in systemic lupus erythematosus: a pilot observational study. Lupus 10, 480–483 (2001).
Wang, H. et al. Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: a prospective multi-centre observational study. Lupus 17, 638–644 (2008).
Zhang, F. S. et al. The efficacy and safety of leflunomide therapy in lupus nephritis by repeat kidney biopsy. Rheumatol. Int. 29, 1331–1335 (2009).
Zhang, M. et al. Leflunomide versus cyclophosphamide in the induction treatment of proliferative lupus nephritis in Chinese patients: a randomized trial. Clin. Rheumatol. 38, 859–867 (2019).
Tam, L.-S. et al. Safety and efficacy of leflunomide in the treatment of lupus nephritis refractory or intolerant to traditional immunosuppressive therapy: an open label trial. Ann. Rheum. Dis. 65, 417–418 (2006).
Singh, H. Rare occurrence of drug induced subacute cutaneous lupus erythematosus with leflunomide therapy. J. Clin. Diagn. Res. 10, OD06 - OD07 (2016).
Marzano, A., Ramoni, S., Del Papa, N., Barbareschi, M. & Alessi, E. Leflunomide-induced subacute cutaneous lupus erythematosus with erythema multiforme-like lesions. Lupus 17, 329–331 (2008).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03866317 (2019).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
Burness, C. B. & Deeks, E. D. Adalimumab: in non-radiographic axial spondyloarthritis. Drugs 72, 2385–2395 (2012).
Goodwin, J. S. & Goodwin, J. M. Failure to recognize efficacious treatments: a history of salicylate therapy in rheumatoid arthritis. Perspect. Biol. Med. 25, 78–92 (1981).
Halford, G. M., Lordkipanidzé, M. & Watson, S. P. 50th anniversary of the discovery of ibuprofen: an interview with Dr Stewart Adams. Platelets 23, 415–422 (2012).
Nash, P. & Clegg, D. O. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann. Rheum. Dis. 64, ii74–ii77 (2005).
Østensen, M. & Villiger, P. M. Nonsteroidal anti-inflammatory drugs in systemic lupus erythematosus. Lupus 9, 566–572 (2000).
Monaco, C., Nanchahal, J., Taylor, P. & Feldmann, M. Anti-TNF therapy: past, present and future. Int. Immunol. 27, 55–62 (2015).
Davis, J. C. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheumatol. 48, 3230–3236 (2003).
Present, D. H. et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 340, 1398–1405 (1999).
Sandborn, W. J. et al. Certolizumab pegol for the treatment of Crohn’s disease. N. Engl. J. Med. 357, 228–238 (2007).
Lipsky, P. E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N. Engl. J. Med. 343, 1594–1602 (2000).
Keystone, E. et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol. 58, 3319–3329 (2008).
Van Der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheumatol. 52, 582–591 (2005).
van der Heijde, D. et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 56, 1498–1509 (2017).
Antoni, C. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann. Rheum. Dis. 64, 1150–1157 (2005).
Mease, P. J. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann. Rheum. Dis. 73, 48–55 (2014).
Weinblatt, M. E. et al. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheumatol. 48, 35–45 (2003).
Smolen, J. S. et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374, 210–221 (2009).
Mease, P. J. et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 52, 3279–3289 (2005).
Kavanaugh, A. et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis. Arthritis Rheumatol. 69, 2151–2161 (2017).
Deodhar, A. et al. Safety and efficacy of golimumab administered intravenously in adults with ankylosing spondylitis: results through week 28 of the GO-ALIVE study. J. Rheumatol. 45, 341–348 (2018).
Revicki, D. A. et al. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and efficacy for ankylosing spondylitis (ATLAS). J. Rheumatol. 35, 1346–1353 (2008).
Blasco-Morente, G., Notario-Ferreira, I., Rueda-Villafranca, B. & Tercedor-Sánchez, J. Subacute cutaneous lupus erythematosus induced by golimumab. Med. Clin. 145, 226–227 (2015).
Swale, V. J., Perrett, C. M., Denton, C. P., Black, C. M. & Rustin, M. H. A. Etanercept-induced systemic lupus erythematosus. Clin. Exp. Dermatol. 28, 604–607 (2003).
Zhu, L. J., Yang, X. & Yu, X. Q. Anti-TNF-α therapies in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 465898 (2010).
Cortés-Hernández, J., Egri, N., Vilardell-Tarrés, M. & Ordi-Ros, J. Etanercept in refractory lupus arthritis: an observational study. Semin. Arthritis Rheumatol. 44, 672–679 (2015).
Goldbach-Mansky, R. Blocking interleukin-1 in rheumatic diseases. Ann. N Y. Acad. Sci. 1182, 111–123 (2009).
Fisher, C. J. et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 (1994).
Bresnihan, B. et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheumatol. 41, 2196–2204 (1998).
Cohen, S. et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 46, 614–624 (2002).
Norheim, K. B., Harboe, E., Gøransson, L. G. & Omdal, R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome-a double blind, randomised clinical trial. PLOS ONE 7, e30123 (2012).
Alten, R. et al. Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet. Disord. 12, 153 (2011).
Ait-Oudhia, S., Lowe, P. J. & Mager, D. E. Bridging clinical outcomes of canakinumab treatment in patients with rheumatoid arthritis with a population model of IL-1β kinetics. CPT Pharmacomet. Syst. Pharmacol. 1, e5 (2012).
Maloney, D. G. et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 90, 2188–2195 (1997).
Protheroe, A., Edwards, J. C. W., Simmons, A., Maclennan, K. & Selby, P. Remission of inflammatory arthropathy in association with anti-CD20 therapy for non-Hodgkin’s lymphoma. Rheumatology 38, 1150–1152 (1999).
Leandro, M. J., Edwards, J. C., Cambridge, G., Ehrenstein, M. R. & Isenberg, D. A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheumatol. 46, 2673–2677 (2002).
Edwards, J. C. W. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00299130 (2017).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00266227 (2013).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00299104 (2017).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00137969 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00282347 (2015).
Looney, R. J. et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheumatol. 50, 2580–2589 (2004).
Leandro, M. J., Cambridge, G., Edwards, J. C., Ehrenstein, M. R. & Isenberg, D. A. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology 44, 1542–1545 (2005).
Tony, H.-P. et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res. Ther. 13, R75 (2011).
Witt, M. et al. Clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE)—results from a nationwide cohort in Germany (GRAID). Lupus 22, 1142–1149 (2013).
Sfikakis, P. P. et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheumatol. 52, 501–513 (2005).
Clair, E. W. S. et al. Rituximab therapy for primary Sjögren’s syndrome: an open-label clinical trial and mechanistic analysis. Arthritis Rheumatol. 65, 1097 (2013).
Dass, S. et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 67, 1541–1544 (2008).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00071812 (2013).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00657007 (2013).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00071487 (2013).
Stohl, W. et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging study. J. Rheumatol. 40, 579–589 (2013).
Schiff, M. et al. Efficacy and safety of tabalumab, an anti-BAFF monoclonal antibody, in patients with moderate-to-severe rheumatoid arthritis and inadequate response to TNF inhibitors: results of a randomised, double-blind, placebo-controlled, phase 3 study. RMD Open 1, e000037 (2015).
Merrill, J. T. et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 332–340 (2016).
Isenberg, D. A. et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 323–331 (2016).
van Vollenhoven, R. F., Wax, S., Li, Y. & Tak, P. P. Safety and efficacy of atacicept in combination with rituximab for reducing the signs and symptoms of rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled pilot trial. Arthritis Rheumatol. 67, 2828–2836 (2015).
Ginzler, E. M. et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res. Ther. 14, R33 (2012).
Isenberg, D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 (2015).
Merrill, J. T. et al. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 77, 883–889 (2018).
Merrill, J. T. et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 62, 3077–3087 (2010).
Song, I.-H. et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann. Rheum. Dis. 70, 1108–1110 (2011).
Lekpa, F. K. et al. Lack of efficacy of abatacept in axial spondylarthropathies refractory to tumor-necrosis-factor inhibition. Joint Bone Spine 79, 47–50 (2012).
Meiners, P. M. et al. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study). Ann. Rheum. Dis. 73, 1393–1396 (2014).
Mease, P. J. et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 76, 1550–1558 (2017).
Ding, C. & Jones, G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev. Recent. Clin. Trials 1, 193–200 (2006).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheumatol. 62, 542–552 (2010).
Rovin, B. H. & Parikh, S. V. Lupus nephritis: the evolving role of novel therapeutics. Am. J. Kidney Dis. 63, 677–690 (2014).
Wallace, D. J. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 76, 534–542 (2017).
Sieper, J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057 (2015).
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O. & Dougados, M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100 (2014).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Mease, P. J. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Smolen, J. S. et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 76, 831–839 (2017).
Poddubnyy, D., Hermann, K.-G. A., Callhoff, J., Listing, J. & Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73, 817–823 (2014).
van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72–52ra72 (2010).
Langley, R. G. et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
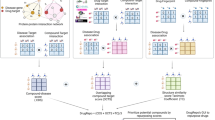
Grammer, A. et al. Drug repositioning in SLE: crowd-sourcing, literature-mining and Big Data analysis. Lupus 25, 1150–1170 (2016).
Grammer, A. C., Ryals, M. M., Catalina, M. D. & Lipsky, P. E. Repositioning drugs for systemic lupus erythematosus. in Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects 567–575 (Academic Press, 2016).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03517722 (2019).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Cingoz, O. Ustekinumab. MAbs 1, 216–221 (2009).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02407223 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03159936 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03502616 (2019).
Wallace, D. J. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392, 222–231 (2018).
Kavanaugh, A. et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J. Rheumatol. 42, 479–488 (2015).
Cutolo, M. et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J. Rheumatol. 43, 1724–1734 (2016).
Edwards, C. J. et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 75, 1065–1073 (2016).
Genovese, M. C. et al. Apremilast in patients with active rheumatoid arthritis: a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol. 67, 1703–1710 (2015).
De Souza, A., Strober, B. E., Merola, J. F., Oliver, S. & Franks, A. G. Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J. Drugs Dermatol. 11, 1224–1226 (2012).
Celgene. Celgene Reports Results from the Phase III POSTURE Study Evaluating Oral OTEZLA® in Ankylosing Spondylitis. https://ir.celgene.com/press-releases/press-release-details/2014/Celgene-Reports-Results-from-the-Phase-III-POSTURE-Study-Evaluating-Oral-OTEZLA-in-Ankylosing-Spondylitis/default.aspx (2014).
Xu, R. & Wang, Q. A genomics-based systems approach towards drug repositioning for rheumatoid arthritis. BMC Genom. 17, 518 (2016).
Labonte, A. C. et al. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLOS ONE 13, e0208132 (2018).
Kingsmore, K. M., Lipsky, P. E. & Grammer, A. C. R. in Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects (ed. Tsokos G. C.) (Elsevier 2016).
Kegerreis, B. et al. Machine learning approaches to predict lupus disease activity from gene expression data. Sci. Rep. 9, 9617 (2019).
Gilson, M. K. et al. BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 44, D1045–D1053 (2016).
Wang, X. et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45, W356–W360 (2017).
Janes, J. et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl Acad. Sci. USA 115, 10750–10755 (2018).
Wu, Z. et al. SDTNBI: an integrated network and chemoinformatics tool for systematic prediction of drug–target interactions and drug repositioning. Brief. Bioinform. 18, bbw012 (2016).
Kuhn, M., von Mering, C., Campillos, M., Jensen, L. J. & Bork, P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 36, D684–D688 (2008).
Szklarczyk, D. et al. STITCH 5: augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380–D384 (2016).
Sun, J., Wu, Y., Xu, H. & Zhao, Z. DTome: a web-based tool for drug-target interactome construction. BMC Bioinform. 13, S7 (2012).
Matsoukas, M.-T. et al. Identification of small-molecule inhibitors of calcineurin-NFATc signaling that mimic the PxIxIT motif of calcineurin binding partners. Sci. Signal. 8, ra63–ra63 (2015).
Rodrigues-Vendramini, F. A. V. et al. Promising new antifungal treatment targeting chorismate synthase from Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 63, e01097–18 (2019).
Latek, D., Rutkowska, E., Niewieczerzal, S. & Cielecka-Piontek, J. Drug-induced diabetes type 2: in silico study involving class B GPCRs. PLOS ONE 14, e0208892 (2019).
Kumar, S. et al. Computational approaches: discovery of GTPase HRas as prospective drug target for 1,3-diazine scaffolds. BMC Chem. 13, 96 (2019).
Li, H. et al. In silico investigation of the pharmacological mechanisms of beneficial effects of Ginkgo biloba L. on Alzheimer’s disease. Nutrients 10, E589 (2018).
Carrella, D. et al. Mantra 2.0: an online collaborative resource for drug mode of action and repurposing by network analysis. Bioinformatics 30, 1787–1788 (2014).
Napolitano, F., Sirci, F., Carrella, D. & di Bernardo, D. Drug-set enrichment analysis: a novel tool to investigate drug mode of action. Bioinformatics 32, 235 (2016).
de Anda-Jáuregui, G., Guo, K., McGregor, B. A. & Hur, J. Exploration of the anti-inflammatory drug space through network pharmacology: applications for drug repurposing. Front. Physiol. 9, 151 (2018).
Brown, A. S. & Patel, C. J. MeSHDD: literature-based drug-drug similarity for drug repositioning. J. Am. Med. Inf. Assoc. 24, 614–618 (2017).
Campillos, M., Kuhn, M., Gavin, A. C., Jensen, L. J. & Bork, P. Drug target identification using side-effect similarity. Science 321, 263–266 (2008).
Kuhn, M., Letunic, I., Jensen, L. J. & Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 44, D1075–D1079 (2016).
Cai, M. C. et al. ADReCS: an ontology database for aiding standardization and hierarchical classification of adverse drug reaction terms. Nucleic Acids Res. 43, D907–D913 (2015).
Huang, L.-H. et al. ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 46, D911–D917 (2018).
Dimitri, G. M. & Lió, P. DrugClust: a machine learning approach for drugs side effects prediction. Comput. Biol. Chem. 68, 204–210 (2017).
Liu, M. et al. Large-scale prediction of adverse drug reactions using chemical, biological, and phenotypic properties of drugs. J. Am. Med. Inf. Assoc. 19, e28–e35 (2012).
Pauwels, E., Stoven, V. & Yamanishi, Y. Predicting drug side-effect profiles: a chemical fragment-based approach. BMC Bioinform. 12, 169 (2011).
Mizutani, S., Pauwels, E., Stoven, V., Goto, S. & Yamanishi, Y. Relating drug–protein interaction network with drug side effects. Bioinformatics 28, i522–i528 (2012).
Bresso, E. et al. Integrative relational machine-learning for understanding drug side-effect profiles. BMC Bioinform. 14, 207 (2013).
Poleksic, A. & Xie, L. Predicting serious rare adverse reactions of novel chemicals. Bioinformatics 34, 2835–2842 (2018).
Zappacosta, A. R. Reversal of baldness in patient receiving minoxidil for hypertension. N. Engl. J. Med. 303, 1480–1481 (1980).
Wang, K., Wan, M., Wang, R.-S. & Weng, Z. Opportunities for web-based drug repositioning: searching for potential antihypertensive agents with hypotension adverse events. J. Med. Internet Res. 18, e76 (2016).
Kvancz, D. A., Sredzinski, M. N. & Tadlock, C. G. Predictive analytics: a case study in machine-learning and claims databases. Am. J. Pharm. Benefits 8, 214–219 (2016).
Doss, J., Mo, H., Carroll, R. J., Crofford, L. J. & Denny, J. C. Phenome-wide association study of rheumatoid arthritis subgroups identifies association between seronegative disease and fibromyalgia. Arthritis Rheumatol. 69, 291–300 (2017).
Gottesman, O. et al. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet. Med. 15, 761–771 (2013).
[No author listed] Sphinx: a resource of the eMERGE Network. https://www.emergesphinx.org (2019).
Denny, J. C. et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat. Biotechnol. 31, 1102–1110 (2013).
International Genetics of Ankylosing Spondylitis Consortium (IGAS) et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013).
Bowes, J. et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat. Commun. 6, 6046 (2015).
Lessard, C. J. et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat. Genet. 45, 1284–1292 (2013).
Li, Y. et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren’s syndrome at 7q11.23. Nat. Genet. 45, 1361–1365 (2013).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464 (2015).
Eyre, S., Orozco, G. & Worthington, J. The genetics revolution in rheumatology: large scale genomic arrays and genetic mapping. Nat. Rev. Rheumatol. 13, 421–432 (2017).
Langefeld, C. D. et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat. Commun. 8, 16021 (2017).
Sun, C. et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat. Genet. 48, 323–330 (2016).
Jarvinen, T. M. et al. Replication of GWAS-identified systemic lupus erythematosus susceptibility genes affirms B-cell receptor pathway signalling and strengthens the role of IRF5 in disease susceptibility in a Northern European population. Rheumatology 51, 87–92 (2012).
Taylor, P. C. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology 58 (Suppl. 1), i17–i26 (2019).
Brown, M. A., Kenna, T. & Wordsworth, B. P. Genetics of ankylosing spondylitis—insights into pathogenesis. Nat. Rev. Rheumatol. 12, 81–91 (2016).
Morris, D. L. et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat. Genet. 48, 940–946 (2016).
Burton, P. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Pritchard, J.-L. E., O’Mara, T. A. & Glubb, D. M. Enhancing the promise of drug repositioning through genetics. Front. Pharmacol. 8, 896 (2017).
Moosavinasab, S. et al. ‘RE:fine drugs’: an interactive dashboard to access drug repurposing opportunities. Database 2016, baw083 (2016).
Martin, P. et al. Chromatin interactions reveal novel gene targets for drug repositioning in rheumatic diseases. Ann. Rheum. Dis. 78, 1127–1134 (2019).
Fang, H. et al. A genetics-led approach defines the drug target landscape of 30 immune-related traits. Nat. Genet. 51, 1082–1091 (2019).
Lamb, J. et al. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Subramanian, A. et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 171, 1437–1452.e17 (2017).
Jia, Z. et al. Cogena, a novel tool for co-expressed gene-set enrichment analysis, applied to drug repositioning and drug mode of action discovery. BMC Genomics 17, 414 (2016).
Chen, Y., Li, Y., Narayan, R., Subramanian, A. & Xie, X. Gene expression inference with deep learning. Bioinformatics 32, 1832–1839 (2016).
Xie, L., He, S., Song, X., Bo, X. & Zhang, Z. Deep learning-based transcriptome data classification for drug-target interaction prediction. BMC Genomics 19, 667 (2018).
Ganter, B. et al. Development of a large-scale chemogenomics database to improve drug candidate selection and to understand mechanisms of chemical toxicity and action. J. Biotechnol. 119, 219–244 (2005).
Ganter, B., Snyder, R. D., Halbert, D. N. & Lee, M. D. Toxicogenomics in drug discovery and development: mechanistic analysis of compound/class-dependent effects using the DrugMatrix® database. Pharmacogenomics 7, 1025–1044 (2006).
Duan, Q. et al. L1000CDS2: LINCS L1000 characteristic direction signatures search engine. NPJ Syst. Biol. Appl. 2, 16015 (2016).
O’Reilly, P. G. et al. QUADrATiC: scalable gene expression connectivity mapping for repurposing FDA-approved therapeutics. BMC Bioinform. 17, 198 (2016).
Grammer, A. C. & Lipsky, P. E. Drug repositioning strategies for the identification of novel therapies for rheumatic autoimmune inflammatory diseases. Rheum. Dis. Clin. N. Am. 43, 467–480 (2017).
Huang, R. et al. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med. 3, 80ps16 (2011).
Corsello, S. M. et al. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat. Med. 23, 405–408 (2017).
Wei, W.-Q. et al. Development and evaluation of an ensemble resource linking medications to their indications. J. Am. Med. Inf. Assoc. 20, 954–961 (2013).
Brown, A. S. & Patel, C. J. A standard database for drug repositioning. Sci. Data 4, 170029 (2017).
Conte, F. et al. A paradigm shift in medicine: a comprehensive review of network-based approaches. Biochim. Biophys. Acta Gene Regul. Mech. 194416 https://doi.org/10.1016/j.bbagrm.2019.194416 (2019).
Vamathevan, J. et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug. Discov. 18, 463–477 (2019).
Ekins, S. et al. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 18, 435–441 (2019).
Gottlieb, A., Stein, G. Y., Ruppin, E. & Sharan, R. PREDICT: a method for inferring novel drug indications with application to personalized medicine. Mol. Syst. Biol. 7, 496 (2011).
Barabási, A.-L., Gulbahce, N. & Loscalzo, J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 12, 56–68 (2011).
Zeng, X. et al. deepDR: a network-based deep learning approach to in silico drug repositioning. Bioinformatics https://doi.org/10.1093/bioinformatics/btz418 (2019).
Guney, E., Menche, J., Vidal, M. & Barábasi, A.-L. Network-based in silico drug efficacy screening. Nat. Commun. 7, 10331 (2016).
Guney, E. Reproducible drug repurposing: when similarity does not suffice. Pac. Symp. Biocomput. 22, 132–143 (2017).
Iskar, M. et al. Characterization of drug-induced transcriptional modules: towards drug repositioning and functional understanding. Mol. Syst. Biol. 9, 662 (2013).
Stohl, W. et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69, 1016–1027 (2017).
Anjorin, A. & Lipsky, P. Engaging African ancestry participants in SLE clinical trials. Lupus Sci. Med. 5, 297 (2018).
Dörner, T. & Lipsky, P. E. Beyond pan-B-cell-directed therapy-new avenues and insights into the pathogenesis of SLE. Nat. Rev. Rheumatol. 12, 645–657 (2016).
Schamberg, J. F. & Wright, C. S. The use of gold and sodium thiosulphate in the treatment of lupus erythematosus. Arch. Derm. Syphilol. 15, 119–137 (1927).
Alderson, H. E. & Way, S. C. Unusual reaction from gold and sodium thiosulphate injection in treatment of lupus erythematosus. Cal. West. Med. 28, 809 (1928).
Monash, S. & Traub, E. F. Modification of therapy with gold compounds in lupus erythematosus. Arch. Derm. Syphilol. 24, 110 (1931).
Finkelstein, A. E. et al. Auranofin. New oral gold compound for treatment of rheumatoid arthritis. Ann. Rheum. Dis. 35, 251–257 (1976).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheumatol. 52, 1756–1765 (2005).
Sinclair, R. J. G. & Duthie, J. J. R. Salazopyrin in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 8, 226–231 (2008).
Kuzell, W. & Gardner, G. Salicylazosulfapyridine (salazopyrin or azopyrin) in rheumatoid arthritis and experimental polyarthritis. Calif. Med. 73, 476–480 (1950).
McConkey, B. et al. Salazopyrin in rheumatoid arthritis. Agents Actions 8, 438–441 (1978).
McConkey, B. et al. Sulphasalazine in rheumatoid arthritis. Br. Med. J. 280, 442–444 (1980).
Skinner, M., Cathcart, E. S., Mills, J. A. & Pinals, R. S. Tetracycline in the treatment of rheumatoid arthritis. A double blind controlled study. Arthritis Rheumatol. 14, 727–732 (1971).
Pullar, T., Hunter, J. A. & Capell, H. A. Sulphasalazine in rheumatoid arthritis: a double blind comparison of sulphasalazine with placebo and sodium aurothiomalate. Br. Med. J. 287, 1102–1104 (1983).
Shippey, E. A., Wagler, V. D. & Collamer, A. N. Hydroxychloroquine: an old drug with new relevance. Cleve. Clin. J. Med. 85, 459–467 (2018).
Bansback, N. et al. Triple therapy versus biologic therapy for active rheumatoid arthritis. Ann. Intern. Med. 167, 8 (2017).
Balak, D. & Hajdarbegovic, E. Drug-induced psoriasis: clinical perspectives. Psoriasis Targets Ther. 7, 87–94 (2017).
Jaffe, I. A. Penicillamine: an anti-rheumatoid drug. Am. J. Med. 75, 63–68 (1983).
Moens, C. Rheumatoid arthritis treated by administration of imuran and actinomycin-c [article in multiple languages]. Arch. Interam. Rheumatol. 8, 278–287 (1965).
Moens, C. & Brocteur, J. Treatment of rheumatoid arthritis with immunosuppressive drugs. I. Clinical study. Acta Rheumatol. Scand. 11, 212–220 (1965).
Black, R. L. et al. Methotrexate therapy in psoriatic arthritis. JAMA 189, 743–747 (1964).
Lorenzen, I. & Videbaek, A. Treatment of collagen diseases with cytostatics. Lancet 2, 558–561 (1965).
Swannell, A. J. & Coomes, E. N. Preliminary results of azathioprine treatment in severe rheumatic disease. Ann. Phys. Med. 10, 112–120 (1969).
Adams, D. A., Gordon, A. & Maxwell, M. H. Azathioprine treatment of immunological renal disease. JAMA 199, 459–463 (1967).
Miescher, P. A. & Riethmueller, D. Diagnosis and treatment of systemic lupus erythematosus. Semin. Hematol. 2, 1–28 (1965).
Sztejnbok, M., Stewart, A., Diamond, H. & Kaplan, D. Azathioprine in the treatment of systemic lupus erythematosus. A controlled study. Arthritis Rheumatol. 14, 639–645 (1971).
Shah, V. V., Lin, E. J., Reddy, S. P. & Wu, J. J. Methotrexate. in Therapy for Severe Psoriasis 37–48 (Elsevier, 2016).
Gordon, D. A. & Urowitz, M. B. Use of azathioprine for rheumatoid arthritis. Arthritis Rheumatol. 25, 1269–1270 (1982).
Austin, H. A. et al. Therapy of lupus nephritis. N. Engl. J. Med. 314, 614–619 (1986).
Felson, D. T. & Anderson, J. Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis. N. Engl. J. Med. 311, 1528–1533 (1984).
Willkens, R. F. Methotrexate treatment of rheumatoid arthritis. Ann. Intern. Med. 103, 612 (1985).
Weinblatt, M. E. Methotrexate: who would have predicted its importance in rheumatoid arthritis? Arthritis Res. Ther. 20, 103 (2018).
Gansauge, S., Breitbart, A., Rinaldi, N. & Schwarz-Eywill, M. Methotrexate in patients with moderate systemic lupus erythematosus (exclusion of renal and central nervous system disease). Ann. Rheum. Dis. 56, 382–385 (1997).
Kingsley, G. H. et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 51, 1368–1377 (2012).
Singh, A. K. & Narsipur, S. S. Cyclosporine: a commentary on brand versus generic formulation exchange. J. Transpl. 2011, 1–6 (2011).
Dooley, M. A. et al. Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J. Am. Soc. Nephrol. 10, 833–839 (1999).
Chan, T. M. et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N. Engl. J. Med. 343, 1156–1162 (2000).
Contreras, G. et al. Sequential therapies for proliferative lupus nephritis. N. Engl. J. Med. 350, 971–980 (2004).
Contreras, G., Tozman, E., Nahar, N. & Metz, D. Maintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus 14, s33–s38 (2005).
Dooley, M. A. et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 365, 1886–1895 (2011).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03865888 (2019).
Asiri, A., Thavaneswaran, A., Kalman-Lamb, G., Chandran, V. & Gladman, D. D. The effectiveness of leflunomide in psoriatic arthritis. Clin. Exp. Rheumatol. 32, 728–731 (2014).
van der Heijde, D. et al. Adalimumab effectively reduces the signs and symptoms of active ankylosing spondylitis in patients with total spinal ankylosis. Ann. Rheum. Dis. 67, 1218–1221 (2007).
Mease, P. et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open 1, e000119 (2015).
Fleischmann, R. M. et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo-controlled trial. Arthritis Rheumatol. 48, 927–934 (2003).
Kremer, J. M. et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 144, 865–876 (2006).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheumatol. 62, 222–233 (2010).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheumatol. 64, 1215–1226 (2012).
AlDhaheri, F. et al. Rituximab can induce remission in a patient with ankylosing spondylitis who failed anti-TNF-α agent. Am. J. Case Rep. 18, 143–147 (2017).
Merrill, J. T. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus. Arthritis Rheumatol. 70, 266–276 (2018).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03312907 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02631538 (2019).
[No authors listed] Atlizumab: anti-IL-6 receptor antibody – Chugai, anti-interleukin-6 receptor antibody – Chugai, MRA – Chugai. BioDrugs 17, 369–372 (2003).
Rovin, B. H. et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 68, 2174–2183 (2016).
McKee, S. Ablynx’ vobarilizumab fails in lupus trial. PharmaTimes http://www.pharmatimes.com/news/ablynx_vobarilizumab_fails_in_lupus_trial_1229404 (2018).
Kauffman, C. L. et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J. Invest. Dermatol. 123, 1037–1044 (2004).
Krueger, G. G. et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N. Engl. J. Med. 356, 580–592 (2007).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 (2008).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Papp, K. A. et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl. J. Med. 366, 1181–1189 (2012).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01059448 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02429882 (2015).
Mease, P. J. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87 (2017).
Lebwohl, M. et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373, 1318–1328 (2015).
van der Heijde, D. et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392, 2441–2451 (2018).
Deodhar, A. et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 71, 599–611 (2019).
Janssen. Janssen Submits Application to U.S. FDA Seeking First-in-Class Approval of TREMFYA® (guselkumab) for Treatment of Adults with Active Psoriatic Arthritis. Janssen.com https://www.janssen.com/janssen-submits-application-us-fda-seeking-first-class-approval-tremfya-guselkumab-treatment-adults (2019).
West, K. CP-690550, a JAK3 inhibitor as an immunosuppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune-mediated disorders. Curr. Opin. Investig. Drugs 10, 491–504 (2009).
Schett, G., Sloan, V. S., Stevens, R. M. & Schafer, P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther. Adv. Musculoskelet. Dis. 2, 271–278 (2010).
Burmester, G. R. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381, 451–460 (2013).
Mease, P. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377, 1537–1550 (2017).
Genovese, M. C. et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374, 1243–1252 (2016).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03843125 (2019).
Smolen, J. S. et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 393, 2303–2311 (2019).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks G. Orozco, E. Guney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Glossary
- In silico
-
A type of biological thought experiment performed on a computer or using specific computer simulation.
- Machine learning
-
An algorithm by which a computer makes predictions based upon learned patterns in the data.
- Pharmacophore
-
The molecular features that are necessary for interactions between a molecule or protein and its ligand.
- Phenome-wide association studies
-
PheWASs. Analysis of the association between disease manifestations, or phenotypes, and a genetic variant.
- Genome-wide association studies
-
GWASs. Analysis of the association between a disease and genetic variants.
- Perturbagens
-
Small molecules, peptides, or genetic modifications that can induce changes in cell types, including cellular phenotypes, protein make-up and gene expression patterns.
- Autoencoder
-
A type of artificial neural network that is used to learn to efficiently classify data in an unsupervised manner.
- Perturbational studies
-
Studies designed to examine the response of cells to various interventions, including drugs and genetic alterations, for the purpose of understanding mechanism of action, changes in phenotype, gene expression or toxicity.
Rights and permissions
About this article
Cite this article
Kingsmore, K.M., Grammer, A.C. & Lipsky, P.E. Drug repurposing to improve treatment of rheumatic autoimmune inflammatory diseases. Nat Rev Rheumatol 16, 32–52 (2020). https://doi.org/10.1038/s41584-019-0337-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0337-0
This article is cited by
-
Computational drug repurposing based on electronic health records: a scoping review
npj Digital Medicine (2022)
-
An introduction to machine learning and analysis of its use in rheumatic diseases
Nature Reviews Rheumatology (2021)
-
The multifaceted functional role of DNA methylation in immune-mediated rheumatic diseases
Clinical Rheumatology (2021)
-
Small-molecule inhibitors get pro-inflammatory TNF into shape
Nature Reviews Rheumatology (2020)
-
Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates
Rheumatology International (2020)