Abstract
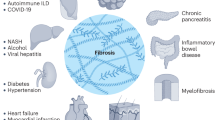
Fibrosis is defined as an excessive deposition of connective tissue components and can affect virtually every organ system, including the skin, lungs, liver and kidney. Fibrotic tissue remodelling often leads to organ malfunction and is commonly associated with high morbidity and mortality. The medical need for effective antifibrotic therapies is thus very high. However, the extraordinarily high costs of drug development and the rare incidence of many fibrotic disorders hinder the development of targeted therapies for individual fibrotic diseases. A potential strategy to overcome this challenge is to target common mechanisms and core pathways that are of central pathophysiological relevance across different fibrotic diseases. The factors influencing susceptibility to and initiation of these diseases are often distinct, with disease-specific and organ-specific risk factors, triggers and sites of first injury. Fibrotic remodelling programmes with shared fibrotic signalling responses such as transforming growth factor-β (TGFβ), platelet-derived growth factor (PDGF), WNT and hedgehog signalling drive disease progression in later stages of fibrotic diseases. The convergence towards shared responses has consequences for drug development as it might enable the development of general antifibrotic compounds that are effective across different disease entities and organs. Technological advances, including new models, single-cell technologies and gene editing, could provide new insights into the pathogenesis of fibrotic diseases and the development of drugs for their treatment.
Key points
-
In fibrotic diseases, disease-specific triggers initiate site-specific injuries, which activate distinct cells that drive fibrosis in a genetically susceptible individual.
-
The inflammatory responses vary across different fibrotic conditions but share polarization towards a T helper 2 cell–M2 macrophage-mediated response, with abundant release of profibrotic mediators as a common feature.
-
Although myofibroblasts are a heterogeneous population of cells that are derived from various cellular precursors, they are activated by a shared set of core pathways, including transforming growth factor-β, platelet-derived growth factor, WNT and hedgehog signalling.
-
Structural changes in fibrotic tissues, such as tissue stiffness and hypoxia, generate an important feed-forward loop that leads to chronicity of tissue-repair responses in fibrotic diseases.
-
The chronic profibrotic milieu induces epigenetic imprinting in myofibroblasts, which serves as a self-amplifying loop to consolidate fibroblast activation in the later stages of fibrotic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thannickal, V. J., Zhou, Y., Gaggar, A. & Duncan, S. R. Fibrosis: ultimate and proximate causes. J. Clin. Invest. 124, 4673–4677 (2014).
Wynn, T. A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Nanthakumar, C. B. et al. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov. 14, 693–720 (2015).
McAnulty, R. J. Fibroblasts and myofibroblasts: their source, function and role in disease. Int. J. Biochem. Cell Biol. 39, 666–671 (2007).
Micallef, L. et al. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 5, S5 (2012).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Santos, A. & Lagares, D. Matrix stiffness: the conductor of organ fibrosis. Curr. Rheumatol. Rep. 20, 2 (2018).
Lokmic, Z., Musyoka, J., Hewitson, T. D. & Darby, I. A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 296, 139–185 (2012).
Beyer, C., Schett, G., Gay, S., Distler, O. & Distler, J. H. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res. Ther. 11, 220 (2009).
Watson, C. J. et al. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum. Mol. Genet. 23, 2176–2188 (2014).
Parker, M. W. et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Invest. 124, 1622–1635 (2014).
Varone, F., Sgalla, G., Iovene, B., Bruni, T. & Richeldi, L. Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Pharmacother. 19, 167–175 (2018).
Roth, G. J. et al. Nintedanib: from discovery to the clinic. J. Med. Chem. 58, 1053–1063 (2015).
Conte, E. et al. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. 58, 13–19 (2014).
Antoniou, K. M., Wuyts, W., Wijsenbeek, M. & Wells, A. U. Medical therapy in idiopathic pulmonary fibrosis. Semin. Respir. Crit. Care Med. 37, 368–377 (2016).
US Food and Drug Administration. FDA approves first treatment for patients with rare type of lung disease (FDA, 2019).
Angiolilli, C. et al. New insights into the genetics and epigenetics of systemic sclerosis. Nat. Rev. Rheumatol. 14, 657–673 (2018).
Kaur, A., Mathai, S. K. & Schwartz, D. A. Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front. Med. 4, 154 (2017).
Barbara, M., Scott, A. & Alkhouri, N. New insights into genetic predisposition and novel therapeutic targets for nonalcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 7, 372–381 (2018).
Tampe, B. & Zeisberg, M. Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol. Dial. Transpl. 29, iv72–iv79 (2014).
Fingerlin, T. E. et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 45, 613–620 (2013).
Allen, R. J. et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir. Med. 5, 869–880 (2017).
Sgalla, G. et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir. Res. 19, 32 (2018).
Heukels, P., Moor, C. C., von der Thusen, J. H., Wijsenbeek, M. S. & Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 147, 79–91 (2019).
Hoffman, T. W., van Moorsel, C. H. M., Borie, R. & Crestani, B. Pulmonary phenotypes associated with genetic variation in telomere-related genes. Curr. Opin. Pulm. Med. 24, 269–280 (2018).
Waters, D. W. et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 315, L162–L172 (2018).
Lehmann, M. et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 50, 1602367 (2017).
Gulati, S. & Thannickal, V. J. The aging lung and idiopathic pulmonary fibrosis. Am. J. Med. Sci. 357, 384–389 (2019).
Povedano, J. M., Martinez, P., Flores, J. M., Mulero, F. & Blasco, M. A. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep. 12, 286–299 (2015).
Al-Issa, K., Tolle, L. B., Purysko, A. S. & Hanouneh, I. A. Short telomere syndrome and fibrosis. QJM 109, 125–126 (2016).
Alder, J. K. et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008).
Donati, B. & Valenti, L. Telomeres, NAFLD and chronic liver disease. Int. J. Mol. Sci. 17, 383 (2016).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Lakota, K. et al. Short lymphocyte, but not granulocyte, telomere length in a subset of patients with systemic sclerosis. Ann. Rheum. Dis. 78, 1142–1144 (2019).
Raschenberger, J. et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci. Rep. 5, 11887 (2015).
Ameh, O. I., Okpechi, I. G., Dandara, C. & Kengne, A. P. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS 21, 143–155 (2017).
Asano, Y. Systemic sclerosis. J. Dermatol. 45, 128–138 (2018).
Matucci-Cerinic, M., Kahaleh, B. & Wigley, F. M. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 65, 1953–1962 (2013).
Lunardi, C. et al. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat. Med. 6, 1183–1186 (2000).
Abraham, D. & Distler, O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res. Ther. 9, S2 (2007).
Winters, N. I., Burman, A., Kropski, J. A. & Blackwell, T. S. Epithelial injury and dysfunction in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Med. Sci. 357, 374–378 (2019).
Mora, A. L., Rojas, M., Pardo, A. & Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 16, 810 (2017).
Wells, A. U., Margaritopoulos, G. A., Antoniou, K. M. & Denton, C. Interstitial lung disease in systemic sclerosis. Semin. Respir. Crit. Care Med. 35, 213–221 (2014).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest. 115, 209–218 (2005).
Qi, R. & Yang, C. Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 9, 1126 (2018).
Kawai, T. & Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21, 317–337 (2009).
Trahtemberg, U. & Mevorach, D. Apoptotic cells induced signaling for immune homeostasis in macrophages and dendritic cells. Front. Immunol. 8, 1356 (2017).
Yu, X., Guo, C., Fisher, P. B., Subjeck, J. R. & Wang, X. Y. Scavenger receptors: emerging roles in cancer biology and immunology. Adv. Cancer Res. 128, 309–364 (2015).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
Malyshev, I. & Malyshev, Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage "switch" phenotype. Biomed. Res. Int. 2015, 341308 (2015).
Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 (2007).
Shook, B., Xiao, E., Kumamoto, Y., Iwasaki, A. & Horsley, V. CD301b+ macrophages are essential for effective skin wound healing. J. Invest. Dermatol. 136, 1885–1891 (2016).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Belperio, J. A. et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 27, 419–427 (2002).
Lee, C. G. et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 194, 809–821 (2001).
Kaviratne, M. et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J. Immunol. 173, 4020–4029 (2004).
Borthwick, L. A. et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 9, 38–55 (2016).
Weng, S. Y. et al. IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. EBioMedicine 29, 92–103 (2018).
Perdiguero, E. G. & Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 17, 2–8 (2016).
Lucas, T. et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184, 3964–3977 (2010).
Gundra, U. M. et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–e122 (2014).
Reyfman, P. A. et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 199, 1517–1536 (2019).
Morse, C. et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 54, 1802441 (2019).
Horsburgh, S., Todryk, S., Ramming, A., Distler, J. H. W. & O'Reilly, S. Innate lymphoid cells and fibrotic regulation. Immunol. Lett. 195, 38–44 (2018).
Zook, E. C. & Kee, B. L. Development of innate lymphoid cells. Nat. Immunol. 17, 775–782 (2016).
Vannella, K. M. et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl Med. 8, 337ra65 (2016).
Hams, E. et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl Acad. Sci. USA 111, 367–372 (2014).
Feghali, C. A. & Wright, T. M. Cytokines in acute and chronic inflammation. Front. Biosci. 2, d12–d26 (1997).
Scotton, C. J. & Chambers, R. C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132, 1311–1321 (2007).
Gustafsson, R., Totterman, T. H., Klareskog, L. & Hallgren, R. Increase in activated T cells and reduction in suppressor inducer T cells in systemic sclerosis. Ann. Rheum. Dis. 49, 40–45 (1990).
Lech, M. & Anders, H. J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 1832, 989–997 (2013).
Bhogal, R. K. & Bona, C. A. B cells: no longer bystanders in liver fibrosis. J. Clin. Invest. 115, 2962–2965 (2005).
Overed-Sayer, C., Rapley, L., Mustelin, T. & Clarke, D. L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 4, 174 (2013).
Mikami, Y., Takada, Y., Hagihara, Y. & Kanai, T. Innate lymphoid cells in organ fibrosis. Cytokine Growth Factor Rev. 42, 27–36 (2018).
Bank, I. The role of γδ T cells in fibrotic diseases. Rambam Maimonides Med. J. 7, e0029 (2016).
Van Linthout, S., Miteva, K. & Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 102, 258–269 (2014).
Oriente, A. et al. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J. Pharmacol. Exp. Ther. 292, 988–994 (2000).
Hashimoto, S., Gon, Y., Takeshita, I., Maruoka, S. & Horie, T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J. Allergy Clin. Immunol. 107, 1001–1008 (2001).
Liu, L. et al. CD4+ T lymphocytes, especially Th2 cells, contribute to the progress of renal fibrosis. Am. J. Nephrol. 36, 386–396 (2012).
Ayano, M. et al. Increased CD226 expression on CD8+ T cells is associated with upregulated cytokine production and endothelial cell injury in patients with systemic sclerosis. J. Immunol. 195, 892–900 (2015).
Li, G. et al. Skin-resident effector memory CD8+CD28− T cells exhibit a profibrotic phenotype in patients with systemic sclerosis. J. Invest. Dermatol. 137, 1042–1050 (2017).
Habiel, D. M. et al. Characterization of CD28null T cells in idiopathic pulmonary fibrosis. Mucosal Immunol. 12, 212–222 (2019).
Luzina, I. G., Todd, N. W., Iacono, A. T. & Atamas, S. P. Roles of T lymphocytes in pulmonary fibrosis. J. Leukoc. Biol. 83, 237–244 (2008).
Dong, Y. et al. Depletion of CD8+ T cells exacerbates CD4+ T cell-induced monocyte-to-fibroblast transition in renal fibrosis. J. Immunol. 196, 1874–1881 (2016).
Wen, Y. et al. Stimulating type 1 angiotensin receptors on T lymphocytes attenuates renal fibrosis. Am. J. Pathol. 189, 981–988 (2019).
Taylor, D. K. et al. T follicular helper-like cells contribute to skin fibrosis. Sci. Transl Med. 10, eaaf5307 (2018).
Brodeur, T. Y. et al. IL-21 promotes pulmonary fibrosis through the induction of profibrotic CD8+ T cells. J. Immunol. 195, 5251–5260 (2015).
Celada, L. J. et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl Med. 10, eaar8356 (2018).
Tsui, J. L. et al. Analysis of pulmonary features and treatment approaches in the COPA syndrome. ERJ Open Res. 4, 00017-2018 (2018).
Peng, X. et al. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J. Pathol. 235, 79–89 (2015).
Meng, F. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776.e3 (2012).
Majd, Z. et al. RORgt inhibition in the liver prevents hepatic fibrosis progression, a proof of concept study with a potent, first in class, hepatocentric RORgt inverse agonist. J. Hepatol. 64, S523 (2016).
Todd, N. W. et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J. Inflamm. Res. 6, 63–70 (2013).
Bosello, S. et al. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res. Ther. 20, 75 (2018).
Matsushita, T. et al. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 54, 192–201 (2006).
Francois, A. et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res. Ther. 15, R168 (2013).
Lee, J. S. et al. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir. Med. 107, 249–255 (2013).
Liaskos, C. et al. Disease-related autoantibody profile in patients with systemic sclerosis. Autoimmunity 50, 414–421 (2017).
Dumoitier, N. et al. Scleroderma peripheral B lymphocytes secrete interleukin-6 and transforming growth factor β and activate fibroblasts. Arthritis Rheumatol. 69, 1078–1089 (2017).
Xue, J. et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J. Immunol. 191, 2089–2095 (2013).
Berger, M. & Steen, V. D. Role of anti-receptor autoantibodies in pathophysiology of scleroderma. Autoimmun. Rev. 16, 1029–1035 (2017).
Svegliati, S. et al. Agonistic anti-PDGF receptor autoantibodies from patients with systemic sclerosis impact human pulmonary artery smooth muscle cells function in vitro. Front. Immunol. 8, 75 (2017).
Baroni, S. S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Riemekasten, G. et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann. Rheum. Dis. 70, 530–536 (2011).
Gunther, J., Rademacher, J., van Laar, J. M., Siegert, E. & Riemekasten, G. Functional autoantibodies in systemic sclerosis. Semin. Immunopathol. 37, 529–542 (2015).
Kim, D. et al. Induction of interferon-α by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-α activity with lung fibrosis. Arthritis Rheum. 58, 2163–2173 (2008).
Vuppalanchi, R. et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol. Int. 6, 379–385 (2012).
Sutti, S. et al. BAFF neutralization ameliorates the evolution of experimental NASH. J. Hepatol. 68, S340 (2018).
Han, H. et al. Renal recruitment of B lymphocytes exacerbates tubulointerstitial fibrosis by promoting monocyte mobilization and infiltration after unilateral ureteral obstruction. J. Pathol. 241, 80–90 (2017).
Raschi, E. et al. Immune complexes containing scleroderma-specific autoantibodies induce a profibrotic and proinflammatory phenotype in skin fibroblasts. Arthritis Res. Ther. 20, 187 (2018).
Kahloon, R. A. et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am. J. Respir. Crit. Care Med. 187, 768–775 (2013).
Li, F. J. et al. Autoimmunity to vimentin is associated with outcomes of patients with idiopathic pulmonary fibrosis. J. Immunol. 199, 1596–1605 (2017).
Schiller, H. B. et al. Deep proteome profiling reveals common prevalence of MZB1-positive plasma B cells in human lung and skin fibrosis. Am. J. Respir. Crit. Care Med. 196, 1298–1310 (2017).
Smith, V. et al. Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann. Rheum. Dis. 69, 193–197 (2010).
Lafyatis, R. et al. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 60, 578–583 (2009).
McGonagle, D. et al. Successful treatment of resistant scleroderma-associated interstitial lung disease with rituximab. Rheumatology 47, 552–553 (2008).
Daoussis, D. et al. Is there a role for B-cell depletion as therapy for scleroderma? A case report and review of the literature. Semin. Arthritis Rheum. 40, 127–136 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03286556 (2019).
Richeldi, L., Davies, H. R., Ferrara, G. & Franco, F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst. Rev. 3, CD002880 (2003).
Raghu, G. et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 178, 948–955 (2008).
Idiopathic Pulmonary Fibrosis Clinical Research Network. et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977 (2012).
Tashkin, D. P. et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 4, 708–719 (2016).
Tashkin, D. P. et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 354, 2655–2666 (2006).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Pope, J. E. et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 44, 1351–1358 (2001).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum. Dis. 77, 212–220 (2018).
Koyama, Y. & Brenner, D. A. Liver inflammation and fibrosis. J. Clin. Invest. 127, 55–64 (2017).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Willis, B. C., duBois, R. M. & Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 3, 377–382 (2006).
Kalluri, R. & Neilson, E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784 (2003).
Yao, L. et al. Paracrine signalling during ZEB1-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ. 26, 943–957 (2019).
Rognoni, E. & Watt, F. M. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 28, 709–722 (2018).
Lim, C. P., Phan, T. T., Lim, I. J. & Cao, X. Cytokine profiling and Stat3 phosphorylation in epithelial-mesenchymal interactions between keloid keratinocytes and fibroblasts. J. Invest. Dermatol. 129, 851–861 (2009).
Marangoni, R. G. et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 67, 1062–1073 (2015).
Mastrogiannaki, M. et al. β-Catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Invest. Dermatol. 136, 1130–1142 (2016).
Sun, C., Berry, W. L. & Olson, L. E. PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144, 83–94 (2017).
McGowan, S. E. & Torday, J. S. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu. Rev. Physiol. 59, 43–62 (1997).
El Agha, E. et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20, 261–273.e3 (2017).
Torday, J. S. & Rehan, V. K. On the evolution of the pulmonary alveolar lipofibroblast. Exp. Cell Res. 340, 215–219 (2016).
El Agha, E. et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141, 296–306 (2014).
Rehan, V. K. & Torday, J. S. PPARγ signaling mediates the evolution, development, homeostasis, and repair of the lung. PPAR Res. 2012, 289867 (2012).
Wei, J. et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLOS ONE 5, e13778 (2010).
Mehal, W. Z., Iredale, J. & Friedman, S. L. Scraping fibrosis: expressway to the core of fibrosis. Nat. Med. 17, 552–553 (2011).
Pannu, J. & Trojanowska, M. Recent advances in fibroblast signaling and biology in scleroderma. Curr. Opin. Rheumatol. 16, 739–745 (2004).
Gyorfi, A. H., Matei, A. E. & Distler, J. H. W. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 68-69, 8–27 (2018).
Distler, J. H. et al. Review: Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis Rheumatol. 69, 257–267 (2017).
Sonnylal, S. et al. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 56, 334–344 (2007).
Lafyatis, R. Transforming growth factor β–at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 10, 706–719 (2014).
Li, M. et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J. Clin. Invest. 121, 277–287 (2011).
Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125, 2795–2807 (2015).
Denton, C. P. et al. Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 56, 323–333 (2007).
Massague, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 (2012).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Blobe, G. C., Schiemann, W. P. & Lodish, H. F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358 (2000).
Robertson, I. B. & Rifkin, D. B. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb. Perspect. Biol. 8, a021907 (2016).
Kim, K. K., Sheppard, D. & Chapman, H. A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 10, a022293 (2018).
Shi, M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011).
Conroy, K. P., Kitto, L. J. & Henderson, N. C. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res. 365, 511–519 (2016).
Reed, N. I. et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl Med. 7, 288ra79 (2015).
Patsenker, E. et al. Pharmacological inhibition of integrin αvβ3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology 50, 1501–1511 (2009).
Horan, G. S. et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Resp. Crit. Care Med. 177, 56–65 (2008).
Hahm, K. et al. αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 170, 110–125 (2007).
Henderson, N. C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Reed, N. I. et al. Exploring N-arylsulfonyl-L-proline scaffold as a platform for potent and selective αvβ1 integrin inhibitors. ACS Med. Chem. Lett. 7, 902–907 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01371305 (2018).
Beyer, C. et al. Stimulation of soluble guanylate cyclase reduces experimental dermal fibrosis. Ann. Rheum. Dis. 71, 1019–1026 (2012).
Beyer, C. et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann. Rheum. Dis. 74, 1408–1416 (2015).
Matei, A. E. et al. Protein kinases G are essential downstream mediators of the antifibrotic effects of sGC stimulators. Ann. Rheum. Dis. 77, 459 (2018).
Knorr, A. et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittelforschung 58, 71–80 (2008).
Wang, Y. et al. Enhancing cGMP in experimental progressive renal fibrosis: soluble guanylate cyclase stimulation vs. phosphodiesterase inhibition. Am. J. Physiol. Ren. Physiol. 290, F167–F176 (2006).
Dou, C. et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 154, 2209–2221.e14 (2018).
Ghosh, A. K. et al. Disruption of transforming growth factor β signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor γ. Arthritis Rheum. 50, 1305–1318 (2004).
Zhu, M. et al. Anti-inflammatory effects of thiazolidinediones in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 45, 111–119 (2011).
Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am. J. Pathol. 174, 519–533 (2009).
Koo, J. B. et al. Anti-fibrogenic effect of PPAR-γ agonists in human intestinal myofibroblasts. BMC Gastroenterol. 17, 73 (2017).
Wei, J. et al. A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses. Ann. Rheum. Dis. 73, 446–454 (2014).
Galli, A. et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology 122, 1924–1940 (2002).
Shiomi, T. et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 106, 3126–3132 (2002).
Kawai, T. et al. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab. Invest. 89, 47–58 (2009).
Erdmann, E., Charbonnel, B. & Wilcox, R. Thiazolidinediones and cardiovascular risk–a question of balance. Curr. Cardiol. Rev. 5, 155–165 (2009).
Grey, A. et al. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 92, 1305–1310 (2007).
Schwartz, A. V. et al. Thiazolidinedione use and bone loss in older diabetic adults. J. Clin. Endocrinol. Metab. 91, 3349–3354 (2006).
Ruzehaji, N. et al. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann. Rheum. Dis. 75, 2175–2183 (2016).
Avouac, J. et al. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Ann. Rheum. Dis. 76, 1931–1940 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02503644 (2019).
Palumbo-Zerr, K. et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat. Med. 21, 62–70 (2015).
Chen, H. Z. et al. The orphan receptor TR3 suppresses intestinal tumorigenesis in mice by downregulating Wnt signalling. Gut 61, 714–724 (2012).
Zerr, P. et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 74, e20 (2015).
Cutolo, M. Further emergent evidence for the vitamin D endocrine system involvement in autoimmune rheumatic disease risk and prognosis. Ann. Rheum. Dis. 72, 473–475 (2013).
Belloli, L., Ughi, N. & Marasini, B. Vitamin D in systemic sclerosis. Clin. Rheumatol. 30, 145–146 (2011).
Calzolari, G., Data, V., Carignola, R. & Angeli, A. Hypovitaminosis D in systemic sclerosis. J. Rheumatol. 36, 2844 (2009).
Caramaschi, P. et al. Very low levels of vitamin D in systemic sclerosis patients. Clin. Rheumatol. 29, 1419–1425 (2010).
Gambichler, T., Chrobok, I., Hoxtermann, S. & Kreuter, A. Significantly decreased serum 25-hydroxyvitamin D levels in a large German systemic sclerosis cohort. J. Rheumatol. 38, 2492–2493 (2011).
Rios Fernandez, R., Fernandez Roldan, C., Callejas Rubio, J. L. & Ortego Centeno, N. Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J. Rheumatol. 37, 1355 (2010).
Zhu, L. D. et al. Spontaneous liver fibrosis induced by long term dietary vitamin D deficiency in adult mice is related to chronic inflammation and enhanced apoptosis. Can. J. Physiol. Pharmacol. 93, 385–394 (2015).
Johnson, L. A., Sauder, K. L., Rodansky, E. S., Simpson, R. U. & Higgins, P. D. R. CARD-024, a vitamin D analog, attenuates the pro-fibrotic response to substrate stiffness in colonic myofibroblasts. Exp. Mol. Pathol. 93, 91–98 (2012).
Yu, R. et al. Protective effects of calcitriol on diabetic nephropathy are mediated by down regulation of TGF-β 1 and CIP4 in diabetic nephropathy rat. Int. J. Clin. Exp. Pathol. 8, 3503–3512 (2015).
Zhang, Z. M. et al. Preventive effects of vitamin D treatment on bleomycin-induced pulmonary fibrosis. Sci. Rep. 5, 17638 (2015).
Wahsh, E., Abu-Elsaad, N., El-Karef, A. & Ibrahim, T. The vitamin D receptor agonist, calcipotriol, modulates fibrogenic pathways mitigating liver fibrosis in-vivo: an experimental study. Eur. J. Pharmacol. 789, 362–369 (2016).
Horn, A. et al. Inhibition of hedgehog signalling prevents experimental fibrosis and induces regression of established fibrosis. Ann. Rheum. Dis. 71, 785–789 (2012).
Lam, A. P. et al. Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Biol. 45, 915–922 (2011).
Horn, A. et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 64, 2724–2733 (2012).
Dees, C. et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann. Rheum. Dis. 70, 1304–1310 (2011).
He, W. et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 (2009).
Konigshoff, M. et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 119, 772–787 (2009).
Guan, S. & Zhou, J. Frizzled-7 mediates TGF-β-induced pulmonary fibrosis by transmitting non-canonical Wnt signaling. Exp. Cell Res. 359, 226–234 (2017).
Saito, A. & Nagase, T. Hippo and TGF-β interplay in the lung field. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L756–L767 (2015).
Burgy, O. & Königshoff, M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 68-69, 67–80 (2018).
Beyer, C. & Distler, J. H. Morphogen pathways in systemic sclerosis. Curr. Rheumatol. Rep. 15, 299 (2013).
Bergmann, C. & Distler, J. H. Canonical Wnt signaling in systemic sclerosis. Lab. Invest. 96, 151–155 (2016).
Beyer, C. et al. Elevated serum levels of sonic hedgehog are associated with fibrotic and vascular manifestations in systemic sclerosis. Ann. Rheum. Dis. 77, 626–628 (2018).
Liang, R. et al. The transcription factor GLI2 as a downstream mediator of transforming growth factor-β-induced fibroblast activation in SSc. Ann. Rheum. Dis. 76, 756–764 (2017).
Hu, B. et al. Reemergence of hedgehog mediates epithelial-mesenchymal crosstalk in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 52, 418–428 (2015).
Ding, H. et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J. Am. Soc. Nephrol. 23, 801–813 (2012).
El-Agroudy, N. N., El-Naga, R. N., El-Razeq, R. A. & El-Demerdash, E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br. J. Pharmacol. 173, 3248–3260 (2016).
Rimkus, T. K., Carpenter, R. L., Qasem, S., Chan, M. & Lo, H. W. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers 8, 22 (2016).
Wei, J. et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy a novel mouse model for scleroderma? Arthritis Rheum. 63, 1707–1717 (2011).
Königshoff, M. et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLOS ONE 3, e2142 (2008).
Cheng, J. H. et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G39–G49 (2008).
He, W. et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl Acad. Sci. USA 107, 21110–21115 (2010).
Trensz, F., Haroun, S., Cloutier, A., Richter, M. V. & Grenier, G. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am. J. Physiol. Cell Physiol. 299, C939–C947 (2010).
Baarsma, H. A. & Konigshoff, M. 'WNT-er is coming': WNT signalling in chronic lung diseases. Thorax 72, 746–759 (2017).
Akhmetshina, A. et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 3, 735 (2012).
Dees, C. et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann. Rheum. Dis. 73, 1232–1239 (2014).
Chen, J. H., Chen, W. L. K., Sider, K. L., Yip, C. Y. Y. & Simmons, C. A. β-Catenin mediates mechanically regulated, transforming growth factor-β 1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 31, 590–597 (2011).
Sato, M. Upregulation of the Wnt/β-catenin pathway induced by transforming growth factor-β in hypertrophic scars and keloids. Acta Derm. Venereol. 86, 300–307 (2006).
Chen, C. W. et al. Pharmacological inhibition of porcupine induces regression of experimental skin fibrosis by targeting Wnt signalling. Ann. Rheum. Dis. 76, 773–778 (2017).
Beyer, C. et al. Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann. Rheum. Dis. 72, 1255–1258 (2013).
Beyer, C. et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann. Rheum. Dis. 71, 761–767 (2012).
Bergmann, C. et al. Inhibition of glycogen synthase kinase 3 β induces dermal fibrosis by activation of the canonical Wnt pathway. Ann. Rheum. Dis. 70, 2191–2198 (2011).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Wei, J. et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 64, 2734–2745 (2012).
Martin-Medina, A. et al. Increased extracellular vesicles mediate Wnt-5a signaling in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.201708-1580OC (2018).
Vuga, L. J. et al. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am. J. Respir. Cell. Mol Biol. 41, 583–589 (2009).
Baarsma, H. A. et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 214, 143–163 (2017).
Blyszczuk, P. et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur. Heart J. 38, 1413–1425 (2017).
Clevers, H., Loh, K. M. & Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014).
Abe, Y. & Tanaka, N. Roles of the hedgehog signaling pathway in epidermal and hair follicle development, homeostasis, and cancer. J. Dev. Biol. 5, 12 (2017).
Konigshoff, M. & Eickelberg, O. WNT signaling in lung disease: a failure or a regeneration signal? Am. J. Respir. Cell Mol. Biol. 42, 21–31 (2010).
Rock, J. & Konigshoff, M. Endogenous lung regeneration: potential and limitations. Am. J. Respir. Crit. Care Med. 186, 1213–1219 (2012).
Zhang, J., Tian, X. J. & Xing, J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J. Clin. Med. 5, 41 (2016).
Borggrefe, T. et al. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia pathways. Biochim. Biophys. Acta 1863, 303–313 (2016).
Cigna, N. et al. The hedgehog system machinery controls transforming growth factor-β-dependent myofibroblastic differentiation in humans: involvement in idiopathic pulmonary fibrosis. Am. J. Pathol. 181, 2126–2137 (2012).
Reyhani, V. et al. PDGF-BB enhances collagen gel contraction through a PI3K-PLCγ-PKC-cofilin pathway. Sci. Rep. 7, 8924 (2017).
Klinkhammer, B. M., Floege, J. & Boor, P. PDGF in organ fibrosis. Mol. Asp. Med. 62, 44–62 (2018).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Demoulin, J. B. & Essaghir, A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 25, 273–283 (2014).
Makino, K. et al. Blockade of PDGF receptors by crenolanib has therapeutic effect in patient fibroblasts and in preclinical models of systemic sclerosis. J. Invest. Dermatol. 137, 1671–1681 (2017).
Lee, J. I. et al. Role of Smad3 in platelet-derived growth factor-C-induced liver fibrosis. Am. J. Physiol. Cell. Physiol. 310, C436–C445 (2016).
Daniels, C. E. et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am. J. Respir. Crit. Care Med. 181, 604–610 (2010).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Hernandez-Rodriguez, N. A. et al. Role of thrombin in pulmonary fibrosis. Lancet 346, 1071–1073 (1995).
Scherlinger, M. et al. Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmun. Rev. 17, 625–635 (2018).
Cloutier, N. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl Acad. Sci. USA 115, E1550–E1559 (2018).
Stachow, A., Jablonska, S. & Skiendzielewska, A. Biogenic amines derived from tryptophan in systemic and cutaneous scleroderma. Acta Derm. Venereol. 59, 1–5 (1979).
Dees, C. et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 208, 961–972 (2011).
Lofdahl, A. et al. 5-HT2B receptor antagonists attenuate myofibroblast differentiation and subsequent fibrotic responses in vitro and in vivo. Physiol. Rep. 4, e12873 (2016).
El-Tanbouly, D. M., Wadie, W. & Sayed, R. H. Modulation of TGF-β/Smad and ERK signaling pathways mediates the anti-fibrotic effect of mirtazapine in mice. Toxicol. Appl. Pharmacol. 329, 224–230 (2017).
Chen, C. et al. Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-β1/Smad3-mediated fibrotic responses through 5-HT2A receptors. Mol. Cell. Biochem. 397, 267–276 (2014).
Hutcheson, J. D., Ryzhova, L. M., Setola, V. & Merryman, W. D. 5-HT2B antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J. Mol. Cell. Cardiol. 53, 707–714 (2012).
Rouzaud-Laborde, C. et al. Platelet activation and arterial peripheral serotonin turnover in cardiac remodeling associated to aortic stenosis. Am. J. Hematol. 90, 15–19 (2015).
Tu, X. et al. Anti-inflammatory renoprotective effect of clopidogrel and irbesartan in chronic renal injury. J. Am. Soc. Nephrol. 19, 77–83 (2008).
Nurden, A. T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 105, S13–S33 (2011).
Walther, D. J. et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell 115, 851–862 (2003).
Yabanoglu, S. et al. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J. Mol. Cell. Cardiol. 46, 518–525 (2009).
Distler, O. et al. The serotonin receptor 2 inhibitor terguride has beneficial effects on skin fibrosis: results from a phase 2 proof of concept study [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 970 (2016).
Ebrahimkhani, M. R. et al. Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease. Nat. Med. 17, 1668–1673 (2011).
Konigshoff, M. et al. Increased expression of 5-hydroxytryptamine2A/B receptors in idiopathic pulmonary fibrosis: a rationale for therapeutic intervention. Thorax 65, 949–955 (2010).
Kim, D. C. et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 33, 535–543 (2013).
Ohba, T. et al. Scleroderma bronchoalveolar lavage fluid contains thrombin, a mediator of human lung fibroblast proliferation via induction of platelet-derived growth factor alpha-receptor. Am. J. Respir. Cell Mol. Biol. 10, 405–412 (1994).
Kitasato, L. et al. Factor Xa in mouse fibroblasts may induce fibrosis more than thrombin. Int. Heart J. 55, 357–361 (2014).
Stetina, R., Votruba, I., Holy, A. & Merta, A. The effect of purine phosphonomethoxyalkyl derivatives on DNA synthesis in CHO Chinese hamster cells. Neoplasma 41, 61–66 (1994).
Chambers, R. C., Leoni, P., Blanc-Brude, O. P., Wembridge, D. E. & Laurent, G. J. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J. Biol. Chem. 275, 35584–35591 (2000).
Deng, X., Mercer, P. F., Scotton, C. J., Gilchrist, A. & Chambers, R. C. Thrombin induces fibroblast CCL2/JE production and release via coupling of PAR1 to Galphaq and cooperation between ERK1/2 and Rho kinase signaling pathways. Mol. Biol. Cell 19, 2520–2533 (2008).
Bogatkevich, G. S., Ludwicka-Bradley, A. & Silver, R. M. Dabigatran, a direct thrombin inhibitor, demonstrates antifibrotic effects on lung fibroblasts. Arthritis Rheum. 60, 3455–3464 (2009).
Dong, A. et al. Direct thrombin inhibition with dabigatran attenuates pressure overload-induced cardiac fibrosis and dysfunction in mice. Thromb. Res. 159, 58–64 (2017).
Duplantier, J. G. et al. A role for thrombin in liver fibrosis. Gut 53, 1682–1687 (2004).
Mora, A. L., Bueno, M. & Rojas, M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J. Clin. Invest. 127, 405–414 (2017).
Hecker, L., Cheng, J. & Thannickal, V. J. Targeting NOX enzymes in pulmonary fibrosis. Cell. Mol. Life Sci. 69, 2365–2371 (2012).
Liu, X. & Chen, Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J. Transl. Med. 15, 207 (2017).
Kavian, N. et al. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 9, 1896 (2018).
Rufini, A., Tucci, P., Celardo, I. & Melino, G. Senescence and aging: the critical roles of p53. Oncogene 32, 5129–5143 (2013).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14, 501–513 (2004).
Armanios, M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. 730, 52–58 (2012).
Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 (2017).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol. 6, 2853–2868 (2008).
Korolchuk, V. I., Miwa, S., Carroll, B. & von Zglinicki, T. Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine 21, 7–13 (2017).
Hoare, M. et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 18, 979–992 (2016).
Razdan, N., Vasilopoulos, T. & Herbig, U. Telomere dysfunction promotes transdifferentiation of human fibroblasts into myofibroblasts. Aging Cell 17, e12838 (2018).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Wells, R. G. Tissue mechanics and fibrosis. Biochim. Biophys. Acta 1832, 884–890 (2013).
Darby, I. A. & Hewitson, T. D. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 365, 553–562 (2016).
van Putten, S., Shafieyan, Y. & Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 93, 133–142 (2016).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Liang, M. et al. Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. J. Am. Soc. Nephrol. 28, 3278–3290 (2017).
Chen, H. et al. Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat. Commun. 7, 12564 (2016).
Wenger, R. H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 (2002).
Distler, J. H. et al. Physiologic responses to hypoxia and implications for hypoxia-inducible factors in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 50, 10–23 (2004).
Corpechot, C. et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35, 1010–1021 (2002).
Orphanides, C., Fine, L. G. & Norman, J. T. Hypoxia stimulates proximal tubular cell matrix production via a TGF-β1-independent mechanism. Kidney Int. 52, 637–647 (1997).
Altorok, N., Tsou, P. S., Coit, P., Khanna, D. & Sawalha, A. H. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann. Rheum. Dis. 74, 1612–1620 (2015).
Wang, Y., Fan, P. S. & Kahaleh, B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 54, 2271–2279 (2006).
Mann, J. et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 14, 275–285 (2007).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
King, T. E., Pardo, A. & Selman, M. Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 (2011).
Kato, M. et al. TGF-β activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat. Cell Biol. 11, 881–889 (2009).
Montgomery, R. L. et al. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 6, 1347–1356 (2014).
Razin, A. & Riggs, A. D. DNA methylation and gene function. Science 210, 604–610 (1980).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Bergmann, C. & Distler, J. H. W. Epigenetic factors as drivers of fibrosis in systemic sclerosis. Epigenomics 9, 463–477 (2017).
Chen, X. et al. Suppression of SUN2 by DNA methylation is associated with HSCs activation and hepatic fibrosis. Cell Death Dis. 9, 1021 (2018).
Sanders, Y. Y. et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 39, 610–618 (2008).
Zhang, Y. et al. Poly(ADP-ribose) polymerase-1 regulates fibroblast activation in systemic sclerosis. Ann. Rheum. Dis. 77, 744–751 (2018).
Noda, S. et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat. Commun. 5, 5797 (2014).
Asano, Y., Bujor, A. M. & Trojanowska, M. The impact of Fli1 deficiency on the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 59, 153–162 (2010).
Asano, Y. & Trojanowska, M. Fli1 represses transcription of the human alpha2(I) collagen gene by recruitment of the HDAC1/p300 complex. PLOS ONE 8, e74930 (2013).
Dees, C. et al. TGFβ stimulates promoter hypermethylation and subsequent silencing of the anti-fibrotic gene Socs3 [abstract 1265]. Arthritis Rheumatol. 60, S1031 (2009).
Asano, Y., Czuwara, J. & Trojanowska, M. Transforming growth factor-β regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J. Biol. Chem. 282, 34672–34683 (2007).
Zhao, S., Cao, M., Wu, H., Hu, Y. & Xue, X. 5-aza-2'-deoxycytidine inhibits the proliferation of lung fibroblasts in neonatal rats exposed to hyperoxia. Pediatr. Neonatol. 58, 122–127 (2017).
Vettori, S., Gay, S. & Distler, O. Role of microRNAs in fibrosis. Open Rheumatol. J. 6, 130–139 (2012).
Liu, G. et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Zhong, X., Chung, A. C. K., Chen, H. Y., Meng, X. M. & Lan, H. Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 22, 1668–1681 (2011).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008).
Maurer, B. et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 (2010).
van Rooij, E. et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105, 13027–13032 (2008).
Wang, B. et al. Suppression of microRNA-29 expression by TGF-β 1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 23, 252–265 (2012).
Pogribny, I. P. et al. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab. Invest. 90, 1437–1446 (2010).
Messemaker, T. C. et al. Antisense long non-coding RNAs are deregulated in skin tissue of patients with systemic sclerosis. J. Invest. Dermatol. 138, 826–835 (2018).
Whitfield, M. L. et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl Acad. Sci. USA 100, 12319–12324 (2003).
Gardner, H. et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 54, 1961–1973 (2006).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLOS ONE 3, e2696 (2008).
Pendergrass, S. A. et al. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J. Invest. Dermatol. 132, 1363–1373 (2012).
Hinchcliff, M. et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J. Invest. Dermatol. 133, 1979–1989 (2013).
Assassi, S. et al. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol. 67, 3016–3026 (2015).
Sargent, J. L. et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J. Invest. Dermatol. 130, 694–705 (2010).
Johnson, M. E. et al. Experimentally-derived fibroblast gene signatures identify molecular pathways associated with distinct subsets of systemic sclerosis patients in three independent cohorts. PLOS ONE 10, e0114017 (2015).
Rice, L. M. et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 67, 3004–3015 (2015).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Whitfield, M. L. et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell 13, 1977–2000 (2002).
Franks, J. M. et al. A machine learning classifier for assigning individual patients with systemic sclerosis to intrinsic molecular subsets. Arthritis Rheumatol. 71, 1701–1710 (2019).
Greenblatt, M. B. et al. Interspecies comparison of human and murine scleroderma reveals IL-13 and CCL2 as disease subset-specific targets. Am. J. Pathol. 180, 1080–1094 (2012).
Chung, L. et al. Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheum. 60, 584–591 (2009).
Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLOS Comput. Biol. 11, e1004005 (2015).
Yang, I. V. et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 68, 1114–1121 (2013).
Lofgren, S. et al. Integrated, multicohort analysis of systemic sclerosis identifies robust transcriptional signature of disease severity. JCI Insight 1, e89073 (2016).
Pendergrass, S. A. et al. Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLOS ONE 5, e12106 (2010).
Derrett-Smith, E. C. et al. Limited cutaneous systemic sclerosis skin demonstrates distinct molecular subsets separated by a cardiovascular development gene expression signature. Arthritis Res. Ther. 19, 156 (2017).
Taroni, J. N. et al. Molecular characterization of systemic sclerosis esophageal pathology identifies inflammatory and proliferative signatures. Arthritis Res. Ther. 17, 194 (2015).
Taroni, J. N. et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 9, 27 (2017).
Chakravarty, E. F. et al. Gene expression changes reflect clinical response in a placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Arthritis Res. Ther. 17, 159 (2015).
Khanna, D. et al. Abatacept in early diffuse cutaneous systemic sclerosis – results of a phase 2 investigator-initiated, multicenter, double-blind randomized placebo-controlled trial. Arthritis Rheumatol. https://doi.org/10.1002/art.41055 (2019).
Martyanov, V. et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLOS ONE 12, e0187580 (2017).
Gordon, J. et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 24-month open label, extension phase, single-centre trial. Clin. Exp. Rheumatol. 32, S-189–S-193 (2014).
Gordon, J. K. et al. Nilotinib (Tasigna) in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial. Arthritis Res. Ther. 17, 213 (2015).
Leask, A. Toward personalized medicine in scleroderma: classification of scleroderma patients into stable "inflammatory" and "fibrotic" subgroups. J. Invest. Dermatol. 132, 1329–1331 (2012).
Martyanov, V. & Whitfield, M. L. Molecular stratification and precision medicine in systemic sclerosis from genomic and proteomic data. Curr. Opin. Rheumatol. 28, 83–88 (2016).
Franks, J. et al. Machine learning classification of peripheral blood gene expression identifies a subset of patients with systemic sclerosis most likely to show clinical improvement in response to hematopoietic stem cell transplant [abstract]. Arthritis Rheumatol. 70 (Suppl. 10), 1876 (2018).
Matei, A. E. et al. Vascularised human skin equivalents as a novel in vitro model of skin fibrosis and platform for testing of antifibrotic drugs. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216108 (2019).
Shultz, L. D. et al. Humanized mouse models of immunological diseases and precision medicine. Mamm. Genome 30, 123–142 (2019).
Lehmann, M. et al. Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir. Res. 19, 175 (2018).
Alsafadi, H. N. et al. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L896–L902 (2017).
Wu, X. et al. Precision-cut human liver slice cultures as an immunological platform. J. Immunol. Methods 455, 71–79 (2018).
Petrovski, S. et al. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 196, 82–93 (2017).
Wang, H., La Russa, M. & Qi, L. S. CRISPR/Cas9 in genome editing and beyond. Annu. Rev. Biochem. 85, 227–264 (2016).
Cai, L., Fisher, A. L., Huang, H. & Xie, Z. CRISPR-mediated genome editing and human diseases. Genes Dis. 3, 244–251 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03545815 (2018).
Tabib, T., Morse, C., Wang, T., Chen, W. & Lafyatis, R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J. Invest. Dermatol. 138, 802–810 (2018).
Lindeman, I. & Stubbington, M. J. T. Antigen receptor sequence reconstruction and clonality inference from scRNA-Seq data. Methods Mol. Biol. 1935, 223–249 (2019).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Peterson, V. M. et al. Multiplexed quantification of proteins and transcripts in single cells. Nat. Biotechnol. 35, 936–939 (2017).
Han, G., Spitzer, M. H., Bendall, S. C., Fantl, W. J. & Nolan, G. P. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat. Protoc. 13, 2121–2148 (2018).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015).
Bushati, N., Smith, J., Briscoe, J. & Watkins, C. An intuitive graphical visualization technique for the interrogation of transcriptome data. Nucleic Acids Res. 39, 7380–7389 (2011).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Lacouture, M. E. et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 64, 437–446 (2015).
Skronska-Wasek, W., Gosens, R., Konigshoff, M. & Baarsma, H. A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 187, 150–166 (2018).
Martinez, F. J. et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 3, 17074 (2017).
Chizzolini, C. et al. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor α. Arthritis Rheum. 48, 2593–2604 (2003).
Boin, F. et al. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis Rheum. 58, 1165–1174 (2008).
Brembilla, N. C. et al. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res. Ther. 15, R151 (2013).
Truchetet, M. E. et al. Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheum. 65, 1347–1356 (2013).
Truchetet, M. E., Brembilla, N. C., Montanari, E., Allanore, Y. & Chizzolini, C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res. Ther. 13, R166 (2011).
Zhang, J. et al. Profibrotic effects of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L487–L497 (2019).
Yang, X., Yang, J., Xing, X., Wan, L. & Li, M. Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res. Ther. 16, R4 (2014).
Antiga, E. et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br. J. Dermatol. 162, 1056–1063 (2010).
MacDonald, K. G. et al. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J. Allergy Clin. Immunol. 135, 946–955.e9 (2015).
Slobodin, G. et al. Regulatory T cells (CD4+CD25brightFoxP3+) expansion in systemic sclerosis correlates with disease activity and severity. Cell. Immunol. 261, 77–80 (2010).
Kotsianidis, I. et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 179, 1121–1130 (2009).
Tanaka, C. et al. Inducible costimulator ligand regulates bleomycin-induced lung and skin fibrosis in a mouse model independently of the inducible costimulator/inducible costimulator ligand pathway. Arthritis Rheum. 62, 1723–1732 (2010).
Fuschiotti, P., Larregina, A. T., Ho, J., Feghali-Bostwick, C. & Medsger, T. A. Jr. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 65, 236–246 (2013).
Daniil, Z. et al. CD8+ T lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis. Respir. Res. 6, 81 (2005).
Acknowledgements
The authors’ research is supported by the grants DI 1537/7-1, DI 1537/8-1, DI 1537/9-1 DI 153/9-2, DI 1537/11-1, DI 1537/12-1, DI 1537/13-1 and DI 1537/14-1 of the German Research Foundation, SFB CRC1181 (project C01) and SFB TR221/ project number 324392634 (B04) of the German Research Foundation and a Career Support Award of Medicine of the Ernst Jung Foundation.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed to writing and review/editing of the manuscript before submission and contributed to discussion of the content.
Corresponding author
Ethics declarations
Competing interests
J.H.W.D. declares that he has consultancy relationships and/or has received research funding from Actelion, Active Biotech, Array Biopharma, Bayer Pharma, Boehringer Ingelheim, BMS, Celgene, GSK, JB Therapeutics, Novartis, Sanofi-Aventis and UCB in the area of potential treatments for systemic sclerosis, and owns stock in 4D Science GmbH. M.R. declares that she is an employee of Boehringer-Ingelheim. The other authors declare no competing interests.
Additional information
Publisher’s note
Nature Reviews Rheumatology thanks A. Wells and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Epigenetic modifications
-
Heritable differences in gene expression that are not encoded by changes of the nucleotide sequence.
- Mitogens
-
Chemical substances that induce cell division.
- Mesangial cells
-
Specialized cells that form the renal mesangium.
- Senolytic therapies
-
Drugs that selectively induce the death of senescent cells.
- Mitophagy
-
The selective removal of mitochondria by autophagy.
- CpG islands
-
Regions of DNA with a high frequency of cytosine–guanine dinucleotides.
- Antagomirs
-
Chemically modified oligonucleotides that are used to silence microRNAs by binding specifically to particular microRNAs.
- Keratoacanthomas
-
Benign tumours of the skin, originating from the hair follicle.
Rights and permissions
About this article
Cite this article
Distler, J.H.W., Györfi, AH., Ramanujam, M. et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 15, 705–730 (2019). https://doi.org/10.1038/s41584-019-0322-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0322-7
This article is cited by
-
Mechanisms of 5-HT receptor antagonists in the regulation of fibrosis in a 3D human liver spheroid model
Scientific Reports (2024)
-
Endogenous stimuli-responsive separating microneedles to inhibit hypertrophic scar through remodeling the pathological microenvironment
Nature Communications (2024)
-
Ketotifen directly modifies the fibrotic response of human skin fibroblasts
Scientific Reports (2024)
-
Lokalisierte Sklerodermie
Die Dermatologie (2024)
-
Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances
Journal of Translational Medicine (2023)