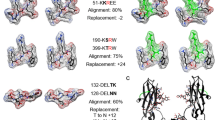
Abstract
In this Perspectives article, we outline a proposed model for understanding the specificity and function of anti-citrullinated protein antibodies (ACPAs). We suggest that ACPAs vary in specificity between two extremes: some are ‘promiscuous’ in that they are highly specific for the citrulline side chain, but cross-react with a range of citrullinated peptides, whereas others are ‘private’ in that their recognition of citrulline as well as proximal amino acid side chains enables protein-specific interactions. Promiscuous ACPAs tend to dominate in the sera both before and after the onset of rheumatoid arthritis, but their functional role has not been clarified. No firm evidence exists that these ACPAs are pathogenic. By contrast, private ACPAs encompass antibodies that specifically recognize citrullinated epitopes on joint proteins or that cross-react with joint proteins, thereby opening up the possibility that these private ACPAs are arthritogenic. These joint-reactive antibodies are more likely to target joints by binding to joint tissues and to promote the formation of local immune complexes leading to bone erosions, pain and arthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rose, H. M. & Ragan, C. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proc. Soc. Exp. Biol. Med. 68, 1–6 (1948).
Schellekens, G. A., de Jong, B. A., van den Hoogen, F. H., van de Putte, L. B. & van Venrooij, W. J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 101, 273–281 (1998).
Girbal-Neuhauser, E. et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J. Immunol. 162, 585–594 (1999).
Aho, K. et al. When does rheumatoid disease start? Arthritis Rheum. 28, 485–489 (1985).
Aho, K. et al. Antifilaggrin antibodies within “normal” range predict rheumatoid arthritis in a linear fashion. J. Rheumatol. 27, 2743–2746 (2000).
Rantapaa-Dahlqvist, S. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
De Rycke, L. et al. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann. Rheum. Dis. 63, 1587–1593 (2004).
Vossenaar, E. R. et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 6, R142–R150 (2004).
Kinloch, A. et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 7, R1421–R1429 (2005).
Lundberg, K. et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 58, 3009–3019 (2008).
Burkhardt, H. et al. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur. J. Immunol. 35, 1643–1652 (2005).
Randen, I. et al. Rheumatoid factor V genes from patients with rheumatoid arthritis are diverse and show evidence of an antigen-driven response. Immunol. Rev. 128, 49–71 (1992).
Holmdahl, R., Malmstrom, V. & Burkhardt, H. Autoimmune priming, tissue attack and chronic inflammation - the three stages of rheumatoid arthritis. Eur. J. Immunol. 44, 1593–1599 (2014).
Shi, J. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl Acad. Sci. USA 108, 17372–17377 (2011).
van de Stadt, L. A. et al. Monoclonal anti-citrullinated protein antibodies selected on citrullinated fibrinogen have distinct targets with different cross-reactivity patterns. Rheumatology 52, 631–635 (2013).
Corsiero, E. et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 75, 1866–1875 (2016).
Steen, J. et al. Recognition of amino acid motifs, rather than specific proteins, by human plasma cell-derived monoclonal antibodies to posttranslationally modified proteins in rheumatoid arthritis. Arthritis Rheumatol. 71, 196–209 (2019).
Vergroesen, R. D. et al. B cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N-glycosylation sites during somatic hypermutation. Ann. Rheum. Dis. 77, 956–958 (2018).
Ge, C. et al. Structural basis of cross-reactivity of anti-citrullinated protein antibodies. Arthritis Rheumatol. 71, 210–221 (2019).
Takizawa, Y. et al. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann. Rheum. Dis. 65, 1013–1020 (2006).
Trier, N. H. et al. Physical characteristics of a citrullinated pro-filaggrin epitope recognized by anti-citrullinated protein antibodies in rheumatoid arthritis sera. PLOS ONE 11, e0168542 (2016).
Cornillet, M. et al. Seropositivity and antibody profiling of patients are dramatically impacted by the features of peptides used as immunosorbents: a lesson from anti-citrullinated protein/peptide antibody. J. Immunol. 201, 3211–3217 (2018).
Uysal, H. et al. Antibodies to citrullinated proteins: molecular interactions and arthritogenicity. Immunol. Rev. 233, 9–33 (2010).
Rombouts, Y. et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 75, 578–585 (2016).
Haag, S. et al. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type II collagen. Arthritis Rheumatol. 66, 1440–1449 (2014).
Uysal, H. et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 206, 449–462 (2009).
Ge, C. et al. Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage. JCI Insight 2, 93688 (2017).
Lloyd, K. A. et al. Differential ACPA binding to nuclear antigens reveals a PAD-independent pathway and a distinct subset of acetylation cross-reactive autoantibodies in rheumatoid arthritis. Front. Immunol. 9, 3033 (2018).
Hensvold, A. H. et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann. Rheum. Dis. 74, 375–380 (2015).
Hensvold, A. H. et al. How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann. Rheum. Dis. 76, 119–125 (2017).
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLOS Med. 6, e1 (2009).
Visser, A., Hamza, N., Kroese, F. G. M. & Bos, N. A. Acquiring new N-glycosylation sites in variable regions of immunoglobulin genes by somatic hypermutation is a common feature of autoimmune diseases. Ann. Rheum. Dis. 77, e69 (2018).
Syversen, S. W. et al. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a 10-year prospective study. Ann. Rheum. Dis. 69, 345–351 (2010).
Scherer, H. U. et al. Distinct ACPA fine specificities, formed under the influence of HLA shared epitope alleles, have no effect on radiographic joint damage in rheumatoid arthritis. Ann. Rheum. Dis. 70, 1461–1464 (2011).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Harre, U. et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 6, 6651 (2015).
Nandakumar, K. S. et al. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur. J. Immunol. 37, 2973–2982 (2007).
Krishnamurthy, A. et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 75, 721–729 (2016).
Amara, K. et al. Retraction: monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J. Exp. Med. 216, 245 (2019).
de Hair, M. J. et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 66, 513–522 (2014).
Burgers, L. E., Ten Brinck, R. M. & van der Helm-van Mil, A. H. M. Is joint pain in patients with arthralgia suspicious for progression to rheumatoid arthritis explained by subclinical inflammation? A cross-sectional MRI study. Rheumatology 58, 86–93 (2019).
Bas, D. B. et al. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 64, 3886–3896 (2012).
Rowley, M. J., Nandakumar, K. S. & Holmdahl, R. The role of collagen antibodies in mediating arthritis. Mod. Rheumatol. 18, 429–441 (2008).
Carlsen, S., Nandakumar, K. S. & Holmdahl, R. Type IX collagen deficiency enhances the binding of cartilage-specific antibodies and arthritis severity. Arthritis Res. Ther. 8, R102 (2006).
Nandakumar, K. S. et al. Streptococcal endo-beta-N-acetylglucosaminidase suppresses antibody-mediated inflammation in vivo. Front. Immunol. 9, 1623 (2018).
Holmdahl, R., Rubin, K., Klareskog, L., Larsson, E. & Wigzell, H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 29, 400–410 (1986).
Holmdahl, R., Jonsson, R., Larsson, P. & Klareskog, L. Early appearance of activated CD4+ T lymphocytes and class II antigen-expressing cells in joints of DBA/1 mice immunized with type II collagen. Lab Invest. 58, 53–60 (1988).
Bersellini Farinotti, A. et al. Cartilage binding antibodies induce pain through immune complex mediated stimulation of neurons. J. Exp. Med. https://doi.org/10.1084/jem.20181657 (2019).
Qu, L., Zhang, P., LaMotte, R. H. & Ma, C. Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain Behav. Immun. 25, 1399–1407 (2011).
Qu, L. et al. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J. Neurosci. 32, 9554–9562 (2012).
Wigerblad, G. et al. Correction: autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann. Rheum. Dis. 78, 865 (2019).
Ossipova, E. et al. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis Res. Ther. 16, R167 (2014).
Titcombe, P. J. et al. Pathogenic citrulline-multispecific B cell receptor clades in rheumatoid arthritis. Arthritis Rheumatol. 70, 1933–1945 (2018).
Maccioni, M. et al. Arthritogenic monoclonal antibodies from K/BxN mice. J. Exp. Med. 195, 1071–1077 (2002).
Geng, H. et al. Cartilage oligomeric matrix protein specific antibodies are pathogenic. Arthritis Res. Ther. 14, R191 (2012).
Ohmi, Y. et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 7, 11205 (2016).
Thiele, G. M. et al. Citrullinated mouse collagen administered to DBA/1J mice in the absence of adjuvant initiates arthritis. Int. Immunopharmacol. 13, 424–431 (2012).
Yamakawa, M. et al. Porphyromonas gingivalis infection exacerbates the onset of rheumatoid arthritis in SKG mice. Clin. Exp. Immunol. 186, 177–189 (2016).
Kuhn, K. A. et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J. Clin. Invest. 116, 961–973 (2006).
Shoda, H. et al. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 13, R191 (2011).
Shelef, M. A. et al. Peptidylarginine deiminase 4 contributes to tumor necrosis factor alpha-induced inflammatory arthritis. Arthritis Rheumatol. 66, 1482–1491 (2014).
Arnoux, F. et al. Peptidyl arginine deiminase immunization induces anticitrullinated protein antibodies in mice with particular MHC types. Proc. Natl Acad. Sci. USA 114, E10169–E10177 (2017).
Sandal, I. et al. Bone loss and aggravated autoimmune arthritis in HLA-DRbeta1-bearing humanized mice following oral challenge with Porphyromonas gingivalis. Arthritis Res. Ther. 18, 249 (2016).
Stoop, J. N. et al. Antibodies specific for carbamylated proteins precede the onset of clinical symptoms in mice with collagen induced arthritis. PLOS ONE 9, e102163 (2014).
Dwivedi, N. et al. B cell tolerance to deiminated histones in BALB/c, C57BL/6, and autoimmune-prone mouse strains. Front. Immunol. 8, 362 (2017).
Yamada, H. et al. Cutting edge: B cells expressing cyclic citrullinated peptide-specific antigen receptor are tolerized in normal conditions. J. Immunol. 201, 3492–3496 (2018).
Cao, D. et al. Pathogenic autoreactive B cells are not negatively selected toward matrix protein collagen II. J. Immunol. 187, 4451–4458 (2011).
Dobritzsch, D. et al. Crystal structure of an arthritogenic anticollagen immune complex. Arthritis Rheum. 63, 3740–3748 (2011).
Mo, J. A., Bona, C. A. & Holmdahl, R. Variable region gene selection of immunoglobulin G-expressing B cells with specificity for a defined epitope on type II collagen. Eur. J. Immunol. 23, 2503–2510 (1993).
Nandakumar, K. S., Svensson, L. & Holmdahl, R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am. J. Pathol. 163, 1827–1837 (2003).
Petkova, S. B. et al. Human antibodies induce arthritis in mice deficient in the low-affinity inhibitory IgG receptor Fc gamma RIIB. J. Exp. Med. 203, 275–280 (2006).
Acknowledgements
The work of the authors is supported by grants from the Knut and Alice Wallenberg Foundation, the Swedish Association against Rheumatism, the Swedish Medical Research Council, the Swedish Foundation for Strategic Research and the EU-funded IMI project BeTheCure. We thank C. Svensson, Karolinska Institute, for critical reading and corrections.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, contributed to the discussion of content, wrote, and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors are listed as inventors on a patent application describing the protective effects of ACPA (Holmdahl R, Rispens T, Xu B, Ge C. Antibodies to citrullinated proteins. European Patent office PCT/EP2018/082236)
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, C., Holmdahl, R. The structure, specificity and function of anti-citrullinated protein antibodies. Nat Rev Rheumatol 15, 503–508 (2019). https://doi.org/10.1038/s41584-019-0244-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0244-4
This article is cited by
-
Immune tolerance of citrullinated peptides
Nature Reviews Rheumatology (2024)
-
Seven-chain adaptive immune receptor repertoire analysis in rheumatoid arthritis reveals novel features associated with disease and clinically relevant phenotypes
Genome Biology (2024)
-
Identification of anti-citrullinated osteopontin antibodies and increased inflammatory response by enhancement of osteopontin binding to fibroblast-like synoviocytes in rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
Clinical significance of anti-cyclic citrullinated peptide (anti-CCP) antibodies in rheumatoid arthritis: Literature review
SN Comprehensive Clinical Medicine (2023)
-
A subset of antibodies targeting citrullinated proteins confers protection from rheumatoid arthritis
Nature Communications (2023)