Abstract
Inflammatory arthritis occurs in many diseases and is characterized by joint inflammation and damage. However, the inflammatory state in arthritis is commonly associated with systemic manifestations, which are generally linked to a poor prognosis. The pro-inflammatory cytokine IL-17 functions within a complex network of cytokines and contributes to the pathogenesis of various inflammatory diseases. Three IL-17 inhibitors have already been approved for the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis. After a brief description of IL-17 and its local effects on joints, this Review focuses on the systemic effects of IL-17 in inflammatory arthritis. Increased circulating concentrations of bioactive IL-17 mediate changes in blood vessels, liver and cardiac and skeletal muscles. The effects of IL-17 on vascular and cardiac cells might contribute to the increased risk of cardiovascular events that occurs in all patients with inflammatory disorders. In the liver, IL-17 contributes to the high circulating concentrations of acute-phase proteins, such as C-reactive protein, and the appearance of liver lesions. In skeletal muscle, IL-17 contributes to muscle contractibility defects and weakness. Thus, targeting IL-17 might have beneficial effects at both local and systemic levels, and could also be proposed for the treatment of a wider range of inflammatory diseases.
Key points
-
Inflammatory arthritis is associated with systemic comorbidities that lead to a poor prognosis.
-
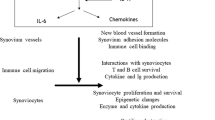
Alone, or in cooperation with other cytokines, IL-17 participates in the establishment and chronicity of several inflammatory diseases, including inflammatory arthritis.
-
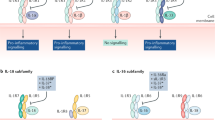
IL-17 can mediate pleiotropic effects throughout the body as IL-17 receptors are expressed on most cell types.
-
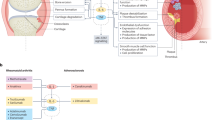
IL-17 contributes to the increased risk of cardiovascular events common to inflammatory diseases by affecting vascular and cardiac cells.
-
IL-17 induces increased concentrations of C-reactive protein and contributes to liver changes by inducing an inflammatory response in liver cells.
-
The systemic effects of IL-17 suggest that targeting this cytokine might be beneficial for the treatment of arthritic and non-arthritic conditions, in addition to inflammatory arthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Symmons, D. P. M. & Gabriel, S. E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 7, 399–408 (2011).
Prasad, M. et al. Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat. Rev. Cardiol. 12, 168–176 (2015).
Crowson, C. S. et al. Rheumatoid arthritis and cardiovascular disease. Am. Heart J. 166, 622–628 (2013).
Beringer, A., Noack, M. & Miossec, P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol. Med. 22, 230–241 (2016).
Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 415–429 (2015).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Robert, M. & Miossec, P. IL-17 in rheumatoid arthritis and precision medicine: from synovitis expression to circulating bioactive levels. Front. Med. 5, 364 (2019).
Onishi, R. M. & Gaffen, S. L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321 (2010).
Kleinschek, M. A. et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170 (2007).
Lavocat, F., Ndongo-Thiam, N. & Miossec, P. Interleukin-25 produced by synoviocytes has anti-inflammatory effects by acting as a receptor antagonist for interleukin-17A function. Front. Immunol. 8, 647 (2017).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Osta, B., Lavocat, F., Eljaafari, A. & Miossec, P. Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front. Immunol. 5, 425 (2014).
Hartupee, J., Liu, C., Novotny, M., Li, X. & Hamilton, T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 179, 4135–4141 (2007).
Hara, M. et al. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J. Surg. Res. 183, 574–582 (2013).
Sparna, T. et al. Genome-wide comparison between IL-17 and combined TNF-alpha/IL-17 induced genes in primary murine hepatocytes. BMC Genomics 11, 226 (2010).
Chabaud, M. et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999).
Leipe, J. et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 62, 2876–2885 (2010).
Raychaudhuri, S. P., Raychaudhuri, S. K. & Genovese, M. C. IL-17 receptor and its functional significance in psoriatic arthritis. Mol. Cell. Biochem. 359, 419–429 (2012).
Hattori, T. et al. Gene expression profiling of IL-17A-treated synovial fibroblasts from the human temporomandibular joint. Mediators Inflamm. 2015, 436067 (2015).
Lee, S.-Y. et al. IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res. Ther. 15, R31 (2013).
Toh, M.-L. et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLOS ONE 5, e13416 (2010).
Benedetti, G., Bonaventura, P., Lavocat, F. & Miossec, P. IL-17A and TNF-α increase the expression of the antiapoptotic adhesion molecule amigo-2 in arthritis synoviocytes. Front. Immunol. 7, 254 (2016).
Agarwal, S., Misra, R. & Aggarwal, A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 35, 515–519 (2008).
Koenders, M. I. et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52, 3239–3247 (2005).
Gravallese, E. M. & Schett, G. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat. Rev. Rheumatol. 14, 631–640 (2018).
Lubberts, E. et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50, 650–659 (2004).
Adamopoulos, I. E. et al. Interleukin-17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res. Ther. 12, R29 (2010).
Yang, L., Zhang, J., Tao, J. & Lu, T. Elevated serum levels of interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS 123, 1025–1031 (2015).
Al-Saadany, H. M., Hussein, M. S., Gaber, R. A. & Zaytoun, H. A. Th-17 cells and serum IL-17 in rheumatoid arthritis patients: Correlation with disease activity and severity. Egypt. Rheumatol. 38, 1–7 (2016).
Gullick, N. J. et al. Enhanced and persistent levels of interleukin (IL)-17+ CD4+ T cells and serum IL-17 in patients with early inflammatory arthritis. Clin. Exp. Immunol. 174, 292–301 (2013).
Lee, Y. H. & Bae, S.-C. Associations between circulating IL-17 levels and rheumatoid arthritis and between IL-17 gene polymorphisms and disease susceptibility: a meta-analysis. Postgrad. Med. J. 93, 465–471 (2017).
Mei, Y. et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin. Rheumatol. 30, 269–273 (2011).
Gullick, N. J. et al. Linking power doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLOS ONE 5, e12516 (2010).
Chen, D.-Y. et al. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res. Ther. 13, R126 (2011).
Taylan, A. et al. Evaluation of the T helper 17 axis in ankylosing spondylitis. Rheumatol. Int. 32, 2511–2515 (2012).
Chen, W.-S. et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J. Chin. Med. Assoc. 75, 303–308 (2012).
Wang, X., Lin, Z., Wei, Q., Jiang, Y. & Gu, J. Expression of IL-23 and IL-17 and effect of IL-23 on IL-17 production in ankylosing spondylitis. Rheumatol. Int. 29, 1343–1347 (2009).
Ndongo-Thiam, N. & Miossec, P. A cell-based bioassay for circulating bioactive IL-17: application to destruction in rheumatoid arthritis. Ann. Rheum. Dis. 74, 1629–1631 (2015).
Kohno, M. et al. Interleukin-17 gene expression in patients with rheumatoid arthritis. Mod. Rheumatol. 18, 15–22 (2008).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Taleb, S., Tedgui, A. & Mallat, Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol. 35, 258–264 (2015).
Robert, M. & Miossec, P. Effects of interleukin 17 on the cardiovascular system. Autoimmun. Rev. 16, 984–991 (2017).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Yuan, S. et al. Interleukin-17 stimulates STAT3-mediated endothelial cell activation for neutrophil recruitment. Cell. Physiol. Biochem. 36, 2340–2356 (2015).
Erbel, C. et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 193, 4344–4355 (2014).
Erbel, C. et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in ApoE-deficient mice. J. Immunol. 183, 8167–8175 (2009).
Eid, R. E. et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119, 1424–1432 (2009).
Hot, A., Lenief, V. & Miossec, P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann. Rheum. Dis. 71, 768–776 (2012).
Zhu, F. et al. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin. Immunol. 141, 152–160 (2011).
Maione, F. et al. IL-17A increases ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun. 408, 658–662 (2011).
Gisterå, A. et al. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl Med. 5, 196ra100 (2013).
Smith, E. et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755 (2010).
Gao, Q. et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 185, 5820–5827 (2010).
Cheng, X. et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis 215, 471–474 (2011).
Madhur, M. S. et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1565–1572 (2011).
Danzaki, K. et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 273–280 (2012).
Taleb, S. et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206, 2067–2077 (2009).
Lee, Y. T. et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 16, 12 (2017).
Liao, Y.-H. et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 59, 420–429 (2012).
Yan, X. et al. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction. J. Am. Heart Assoc. 1, e004408 (2012).
Zhou, S.-F. et al. IL-17A promotes ventricular remodeling after myocardial infarction. J. Mol. Med. 92, 1105–1116 (2014).
Markó, L. et al. Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60, 1430–1436 (2012).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Wu, J. et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 114, 616–625 (2014).
Amador, C. A. et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63, 797–803 (2014).
Liu, Z. et al. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 221, 232–241 (2012).
Erbel, C. et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 106, 125–134 (2011).
Hashmi, S. & Zeng, Q. T. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 17, 699–706 (2006).
Cheng, X. et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 127, 89–97 (2008).
Bochaton, T. et al. Early kinetics of serum Interleukine-17A and infarct size in patients with reperfused acute ST-elevated myocardial infarction. PLOS ONE 12, e0188202 (2017).
Simon, T. et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 34, 570–577 (2013).
Gelfand, J. M. et al. Risk of myocardial infarction in patients with psoriasis. JAMA 296, 1735–1741 (2006).
Gelfand, J. M. et al. The risk of stroke in patients with psoriasis. J. Invest. Dermatol. 129, 2411–2418 (2009).
Ding, H.-S. et al. Interleukin-17 contributes to cardiovascular diseases. Mol. Biol. Rep. 39, 7473–7478 (2012).
Krueger, J. G. & Brunner, P. M. Interleukin-17 alters the biology of many cell types involved in the genesis of psoriasis, systemic inflammation and associated comorbidities. Exp. Dermatol. 27, 115–123 (2018).
Beringer, A. & Miossec, P. IL-17 and IL-17-producing cells and liver diseases, with focus on autoimmune liver diseases. Autoimmun. Rev. 17, 1176–1185 (2018).
Lafdil, F., Miller, A. M., Ki, S. H. & Gao, B. Th17 cells and their associated cytokines in liver diseases. Cell. Mol. Immunol. 7, 250–254 (2010).
Lemmers, A. et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 49, 646–657 (2009).
Meng, F. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776 (2012).
Beringer, A., Thiam, N., Molle, J., Bartosch, B. & Miossec, P. Synergistic effect of interleukin-17 and tumour necrosis factor-α on inflammatory response in hepatocytes through interleukin-6-dependent and independent pathways. Clin. Exp. Immunol. 193, 221–233 (2018).
Gu, F.-M. et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol. Cancer 10, 150 (2011).
Patel, D. N. et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J. Biol. Chem. 282, 27229–27238 (2007).
Zhao, L. et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLOS ONE 6, e18909 (2011).
Pai, J. K. et al. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 351, 2599–2610 (2004).
Tan, Z. et al. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J. Immunol. 191, 1835–1844 (2013).
Sun, H. Q. et al. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J. Viral Hepat. 19, 396–403 (2012).
Harada, K. et al. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin. Exp. Immunol. 157, 261–270 (2009).
Shi, T. et al. The distribution and the fibrotic role of elevated inflammatory Th17 cells in patients with primary biliary cirrhosis. Medicine (Baltimore) 94, e1888 (2015).
Fabre, T., Kared, H., Friedman, S. L. & Shoukry, N. H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 193, 3925–3933 (2014).
Amara, S. et al. Synergistic effect of pro-inflammatory TNFα and IL-17 in periostin mediated collagen deposition: potential role in liver fibrosis. Mol. Immunol. 64, 26–35 (2015).
Lafdil, F. et al. Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology 137, 2125–2135 (2009).
Zhang, Y. et al. MicroRNA let-7a ameliorates Con A-induced hepatitis by inhibiting IL-6-dependent Th17 cell differentiation. J. Clin. Immunol. 33, 630–639 (2013).
Xu, M. et al. Regulation of the development of acute hepatitis by IL-23 through IL-22 and IL-17 production. Eur. J. Immunol. 41, 2828–2839 (2011).
Yan, S., Wang, L., Liu, N., Wang, Y. & Chu, Y. Critical role of interleukin-17/interleukin-17 receptor axis in mediating Con A-induced hepatitis. Immunol. Cell Biol. 90, 421–428 (2012).
He, J., Lang, G., Ding, S. & Li, L. Pathological role of interleukin-17 in poly I:C-induced hepatitis. PLOS ONE 8, e73909 (2013).
Furuya, S. et al. Interleukin 17A plays a role in lipopolysaccharide/D-galactosamine-induced fulminant hepatic injury in mice. J. Surg. Res. 199, 487–493 (2015).
Yu, H. et al. IL-17 contributes to autoimmune hepatitis. J. Huazhong Univ. Sci. Technol. Med. Sci. 30, 443–446 (2010).
Kawata, K. et al. Identification of potential cytokine pathways for therapeutic intervention in murine primary biliary cirrhosis. PLOS ONE 8, e74225 (2013).
van der Voort, E. A. M., Wakkee, M., Veldt-Kok, P., Darwish Murad, S. & Nijsten, T. Enhanced liver fibrosis test in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis: a cross-sectional comparison with procollagen-3 N-terminal peptide (P3NP). Br. J. Dermatol. 176, 1599–1606 (2017).
Ogdie, A. et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J. Invest. Dermatol. 138, 760–767 (2018).
Ruderman, E. M. et al. Histologic liver abnormalities in an autopsy series of patients with rheumatoid arthritis. Br. J. Rheumatol. 36, 210–213 (1997).
Quintin, E., Scoazec, J.-Y., Marotte, H. & Miossec, P. Rare incidence of methotrexate-specific lesions in liver biopsy of patients with arthritis and elevated liver enzymes. Arthritis Res. Ther. 12, R143 (2010).
Toulemonde, G., Scoazec, J.-Y. & Miossec, P. Treatment with etanercept of autoimmune hepatitis associated with rheumatoid arthritis: an open label proof of concept study. Ann. Rheum. Dis. 71, 1423–1424 (2012).
Giles, D. A., Moreno-Fernandez, M. E. & Divanovic, S. IL-17 axis driven inflammation in non-alcoholic fatty liver disease progression. Curr. Drug Targets 16, 1315–1323 (2015).
Chackelevicius, C. M., Gambaro, S. E., Tiribelli, C. & Rosso, N. Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J. Gastroenterol. 22, 9096–9103 (2016).
Chevrel, G. et al. Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J. Neuroimmunol. 137, 125–133 (2003).
Tournadre, A., Lenief, V. & Miossec, P. Expression of Toll-like receptor 3 and Toll-like receptor 7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis Rheum. 62, 2144–2151 (2010).
Beringer, A., Gouriou, Y., Lavocat, F., Michel, O. & Miossec, P. Blockade of store-operated calcium entry reduces IL-17/TNF cytokine-induced inflammatory response in human myoblasts. Front. Immunol. 9, 3170 (2019).
Kocić, J. et al. Interleukin 17 inhibits myogenic and promotes osteogenic differentiation of C2C12 myoblasts by activating ERK1,2. Biochim. Biophys. Acta 1823, 838–849 (2012).
Kocić, J. et al. Interleukin-17 modulates myoblast cell migration by inhibiting urokinase type plasminogen activator expression through p38 mitogen-activated protein kinase. Int. J. Biochem. Cell Biol. 45, 464–475 (2013).
Su, S.-A. et al. Interleukin-17A mediates cardiomyocyte apoptosis through Stat3-iNOS pathway. Biochim. Biophys. Acta 1863, 2784–2794 (2016).
LaFramboise, W. A. et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am. J. Physiol. Cell Physiol. 292, C1799–C1808 (2007).
Wu, L. et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 211, 1449–1464 (2014).
Cortez, D. M. et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 293, H3356–H3365 (2007).
Valente, A. J. et al. Interleukin-17A stimulates cardiac fibroblast proliferation and migration via negative regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cell. Signal. 24, 560–568 (2012).
Atefi, G. et al. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 25, 2500–2508 (2011).
Janssen, S. P. M. et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 111, 996–1005 (2005).
Lazzerini, P. E., Capecchi, P. L. & Laghi-Pasini, F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur. Heart J. 38, 1717–1727 (2017).
Hanaoka, B. Y. et al. Chronic inflammation in RA: mediator of skeletal muscle pathology and physical impairment. Arthritis Care Res. 71, 173–177 (2019).
Huffman, K. M. et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res. Ther. 19, 12 (2017).
Yamada, T., Steinz, M. M., Kenne, E. & Lanner, J. T. Muscle weakness in rheumatoid arthritis: the role of Ca2+ and free radical signaling. EBioMedicine 23, 12–19 (2017).
Genovese, M. C. et al. Safety and efficacy of open-label subcutaneous ixekizumab treatment for 48 weeks in a phase II study in biologic-naive and TNF-IR patients with rheumatoid arthritis. J. Rheumatol. 43, 289–297 (2016).
Blanco, F. J. et al. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 69, 1144–1153 (2017).
Kunwar, S., Dahal, K. & Sharma, S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 36, 1065–1075 (2016).
O’Brien, K. M. et al. IL-17A synergistically enhances bile acid–induced inflammation during obstructive cholestasis. Am. J. Pathol. 183, 1498–1507 (2013).
Acknowledgements
The work of A.B. is supported by the Ministry of Education and Research, France. P.M. is a senior member of the Institut Universitaire de France. His laboratory is supported in part by the IHU OPERA.
Peer review information
Nature Reviews Rheumatology thanks S. Divanovic, P. Wenzel and E. Lubberts for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.B. researched data for the article. P.M. reviewed and/or edited the manuscript before submission. Both authors made a substantial contribution to discussion of content and wrote the article.
Corresponding author
Ethics declarations
Competing interests
P.M. holds a patent on the IL-17 bioassay (patent number 20170176456). A.B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beringer, A., Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat Rev Rheumatol 15, 491–501 (2019). https://doi.org/10.1038/s41584-019-0243-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0243-5
This article is cited by
-
Inhibition of IL-17 signaling in macrophages underlies the anti-arthritic effects of halofuginone hydrobromide: Network pharmacology, molecular docking, and experimental validation
BMC Complementary Medicine and Therapies (2024)
-
Role of Interleukin-17 family cytokines in disease severity of patients with knee osteoarthritis
Advances in Rheumatology (2024)
-
Reactive arthritis following COVID-19 current evidence, diagnosis, and management strategies
Journal of Orthopaedic Surgery and Research (2023)
-
Blocking interleukin-23 ameliorates neuromuscular and thymic defects in myasthenia gravis
Journal of Neuroinflammation (2023)
-
Association of inflammatory biomarkers and disease activity with subclinical myocardial dysfunction in psoriatic arthritis
Scientific Reports (2023)