Abstract
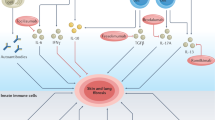
Systemic sclerosis (SSc) is an autoimmune fibrotic disease of unknown aetiology that is characterized by vascular changes in the skin and visceral organs. Autologous haematopoietic stem cell transplantation can improve skin and organ fibrosis in patients with progressive disease and a high risk of organ failure, indicating that cells originating in the bone marrow are important contributors to the pathogenesis of SSc. Animal studies also indicate a pivotal function of myeloid cells in the development of fibrosis leading to changes in the tissue architecture and dysfunction in multiple organs such as the heart, lungs, liver and kidney. In this Review, we summarize current knowledge about the function of myeloid cells in fibrogenesis that occurs in patients with SSc. Targeted therapies currently in clinical studies for SSc might affect myeloid cell-related pathways. Therefore, myeloid cells might be used as cellular biomarkers of disease through the application of high-dimensional techniques such as mass cytometry and single-cell RNA sequencing.
Key points
-
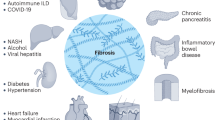
Chronic tissue injury, inflammation and prolonged fibroblast activation lead to fibrosis and organ dysfunction in systemic sclerosis (SSc).
-
Myeloid cells are crucial antigen-presenting cells, regulators of inflammatory responses and producers of cytokines, processes that are implicated in fibrogenesis.
-
Despite comprehensive investigation of myeloid cells, the function of these cells in SSc is not fully understood.
-
High-dimensional analysis of myeloid cells might identify a biomarker of SSc onset and progression.
-
Strategies that target myeloid cell-related pathways might prevent or reverse tissue fibrosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jordan, S., Chung, J. & Distler, O. Preclinical and translational research to discover potentially effective antifibrotic therapies in systemic sclerosis. Curr. Opin. Rheumatol. 25, 679–685 (2013).
Mayes, M. D. et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 48, 2246–2255 (2003).
Peoples, C. et al. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J. Scleroderma Relat. Disord. 1, 177–240 (2016).
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Primers 1, 15002 (2015).
Bujak, M. & Frangogiannis, N. G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 74, 184–195 (2007).
Lech, M. & Anders, H. J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 1832, 989–997 (2013).
Chizzolini, C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Curr. Opin. Rheumatol. 20, 707–712 (2008).
O’Reilly, S., Hugle, T. & van Laar, J. M. T cells in systemic sclerosis: a reappraisal. Rheumatology 51, 1540–1549 (2012).
Sakkas, L. I. & Platsoucas, C. D. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 50, 1721–1733 (2004).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Waldner, H. The role of innate immune responses in autoimmune disease development. Autoimmun. Rev. 8, 400–404 (2009).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Epelman, S., Lavine, K. J. & Randolph, G. J. Origin and functions of tissue macrophages. Immunity 41, 21–35 (2014).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Hoeffel, G. et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Ginhoux, F. & Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016).
Satoh, T. et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 541, 96–101 (2017).
Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 (2011).
Passlick, B., Flieger, D. & Ziegler-Heitbrock, H. W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 (1989).
Wong, K. L. et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118, e16–31 (2011).
Higashi-Kuwata, N. et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res. Ther. 12, R128 (2010).
Lescoat, A. et al. CD16-positive circulating monocytes and fibrotic manifestations of systemic sclerosis. Clin. Rheumatol. 36, 1649–1654 (2017).
Ong, S. M. et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 9, 266 (2018).
Mathai, S. K. et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 90, 812–823 (2010).
Soldano, S. P. et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann. Rheum. Dis. 77, 1842–1845 (2018).
Chia, J. J. & Lu, T. T. Update on macrophages and innate immunity in scleroderma. Curr. Opin. Rheumatol. 27, 530–536 (2015).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404 (2017).
Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Stifano, G. & Christmann, R. B. Macrophage involvement in systemic sclerosis: do we need more evidence? Curr. Rheumatol. Rep. 18, 2 (2016).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Schlitzer, A. & Ginhoux, F. Organization of the mouse and human DC network. Curr. Opin. Immunol. 26, 90–99 (2014).
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012).
Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37, 60–73 (2012).
Schlitzer, A., Zhang, W., Song, M. & Ma, X. Recent advances in understanding dendritic cell development, classification, and phenotype. F1000Res. 7, 1558 (2018).
Segura, E. & Amigorena, S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 34, 440–445 (2013).
Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Affandi, A. J., Carvalheiro, T., Radstake, T. & Marut, W. Dendritic cells in systemic sclerosis: advances from human and mice studies. Immunol. Lett. 195, 18–29 (2018).
Veglia, F. & Gabrilovich, D. I. Dendritic cells in cancer: the role revisited. Curr. Opin. Immunol. 45, 43–51 (2017).
Binai, N., O’Reilly, S., Griffiths, B., van Laar, J. M. & Hugle, T. Differentiation potential of CD14+ monocytes into myofibroblasts in patients with systemic sclerosis. PLOS ONE 7, e33508 (2012).
Dantas, A. T. et al. Reassessing the role of the active TGF-beta1 as a biomarker in systemic sclerosis: association of serum levels with clinical manifestations. Dis. Markers 2016, 6064830 (2016).
Hasegawa, M., Sato, S. & Takehara, K. Augmented production of transforming growth factor-beta by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Arch. Dermatol. Res. 296, 89–93 (2004).
Reese, C. et al. Caveolin-1 deficiency may predispose African Americans to systemic sclerosis-related interstitial lung disease. Arthritis Rheumatol. 66, 1909–1919 (2014).
Tourkina, E. et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair 4, 15 (2011).
Lee, R. et al. Enhanced chemokine-receptor expression, function, and signaling in healthy African American and scleroderma-patient monocytes are regulated by caveolin-1. Fibrogenesis Tissue Repair 8, 11 (2015).
Ciechomska, M. et al. Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann. Rheum. Dis. 72, 1382–1389 (2013).
Visse, R. & Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839 (2003).
Ciechomska, M. et al. Histone demethylation and Toll-like receptor 8-dependent cross-talk in monocytes promotes transdifferentiation of fibroblasts in systemic sclerosis via Fra-2. Arthritis Rheumatol. 68, 1493–1504 (2016).
Maurer, B. et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann. Rheum. Dis. 71, 1382–1387 (2012).
Maurer, B. et al. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation 120, 2367–2376 (2009).
Reich, N. et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum. 62, 280–290 (2010).
Bohgaki, T. et al. Up regulated expression of tumour necrosis factor {alpha} converting enzyme in peripheral monocytes of patients with early systemic sclerosis. Ann. Rheum. Dis. 64, 1165–1173 (2005).
Moss, M. L. & Minond, D. Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm. 2017, 9673537 (2017).
Schumacher, N. et al. Shedding of endogenous interleukin-6 receptor (IL-6R) is governed by a disintegrin and metalloproteinase (ADAM) proteases while a full-length IL-6R isoform localizes to circulating microvesicles. J. Biol. Chem. 290, 26059–26071 (2015).
Duffy, M. J., Crown, J. & Mullooly, M. ADAM10 and ADAM17: new players in trastuzumab tesistance. Oncotarget 5, 10963–10964 (2014).
Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLOS Comput. Biol. 11, e1004005 (2015).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
O’Reilly, S., Cant, R., Ciechomska, M. & van Laar, J. M. Interleukin-6: a new therapeutic target in systemic sclerosis? Clin. Transl Immunol. 2, e4 (2013).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Sahin, U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 (2004).
Midgley, A. C. et al. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 288, 14824–14838 (2013).
Tan, F. K. et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology 45, 694–702 (2006).
Farina, G. A. et al. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J. Invest. Dermatol. 130, 2583–2593 (2010).
Christmann, R. B. et al. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum. 63, 1718–1728 (2011).
York, M. R. et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020 (2007).
Moreno-Moral, A. et al. Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann. Rheum. Dis. 77, 596–601 (2018).
Manetti, M. Deciphering the alternatively activated (M2) phenotype of macrophages in scleroderma. Exp. Dermatol. 24, 576–578 (2015).
Del Galdo, F., Maul, G. G., Jimenez, S. A. & Artlett, C. M. Expression of allograft inflammatory factor 1 in tissues from patients with systemic sclerosis and in vitro differential expression of its isoforms in response to transforming growth factor beta. Arthritis Rheum. 54, 2616–2625 (2006).
Utans, U., Arceci, R. J., Yamashita, Y. & Russell, M. E. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J. Clin. Invest. 95, 2954–2962 (1995).
Otieno, F. G., Lopez, A. M., Jimenez, S. A., Gentiletti, J. & Artlett, C. M. Allograft inflammatory factor-1 and tumor necrosis factor single nucleotide polymorphisms in systemic sclerosis. Tissue Antigens 69, 583–591 (2007).
Yamamoto, A. et al. Allograft inflammatory factor-1 is overexpressed and induces fibroblast chemotaxis in the skin of sclerodermatous GVHD in a murine model. Immunol. Lett. 135, 144–150 (2011).
Rabquer, B. J. et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res. Ther. 13, R18 (2011).
Distler, O. et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 44, 2665–2678 (2001).
Galindo, M. et al. Chemokine expression by systemic sclerosis fibroblasts: abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 44, 1382–1386 (2001).
Yamamoto, T., Eckes, B., Hartmann, K. & Krieg, T. Expression of monocyte chemoattractant protein-1 in the lesional skin of systemic sclerosis. J. Dermatol. Sci. 26, 133–139 (2001).
Ong, V. H. et al. Monocyte chemoattractant protein 3 as a mediator of fibrosis: Overexpression in systemic sclerosis and the type 1 tight-skin mouse. Arthritis Rheum. 48, 1979–1991 (2003).
Bandinelli, F. et al. CCL2, CCL3 and CCL5 chemokines in systemic sclerosis: the correlation with SSc clinical features and the effect of prostaglandin E1 treatment. Clin. Exp. Rheumatol. 30, S44–S49 (2012).
Assassi, S. et al. Skin gene expression correlates of severity of interstitial lung disease in systemic sclerosis. Arthritis Rheum. 65, 2917–2927 (2013).
Carulli, M. T. et al. Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: evidence for autocrine regulation of myofibroblast differentiation. Arthritis Rheum. 52, 3772–3782 (2005).
Abraham, D. J., Krieg, T., Distler, J. & Distler, O. Overview of pathogenesis of systemic sclerosis. Rheumatology 48 (Suppl. 3), iii3–iii7 (2009).
Distler, J. H. et al. Monocyte chemoattractant protein 1 released from glycosaminoglycans mediates its profibrotic effects in systemic sclerosis via the release of interleukin-4 from T cells. Arthritis Rheum. 54, 214–225 (2006).
Zhang, K., Gharaee-Kermani, M., Jones, M. L., Warren, J. S. & Phan, S. H. Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J. Immunol. 153, 4733–4741 (1994).
Ferreira, A. M. et al. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Invest. Dermatol. 126, 1900–1908 (2006).
Distler, J. H., Akhmetshina, A., Schett, G. & Distler, O. Monocyte chemoattractant proteins in the pathogenesis of systemic sclerosis. Rheumatology 48, 98–103 (2009).
Beyer, C., Schett, G., Distler, O. & Distler, J. H. Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 62, 2831–2844 (2010).
Vergunst, C. E. et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939 (2008).
Mathes, A. L. et al. Global chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. Ann. Rheum. Dis. 73, 1864–1872 (2014).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Fasnacht, N. et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J. Exp. Med. 211, 2265–2279 (2014).
Wu, M. et al. Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheumatol. 66, 1010–1021 (2014).
Kato, A. et al. Oligosaccharide modification by N-acetylglucosaminyltransferase-V in macrophages are involved in pathogenesis of bleomycin-induced scleroderma. Exp. Dermatol. 24, 585–590 (2015).
Tian, H. et al. The implication of N-acetylglucosaminyltransferase V expression in gastric cancer. Pathobiology 75, 288–294 (2008).
Demetriou, M., Granovsky, M., Quaggin, S. & Dennis, J. W. Negative regulation of T cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 (2001).
Morgan, R. et al. N-Acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J. Immunol. 173, 7200–7208 (2004).
Knipper, J. A. et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 43, 803–816 (2015).
Martins, V. et al. FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis and lipoatrophy. Am. J. Pathol. 185, 2768–2776 (2015).
Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 174, 519–533 (2009).
Kruglikov, I. L. Interfacial adipose tissue in systemic sclerosis. Curr. Rheumatol. Rep. 19, 4 (2017).
Khanna, D. et al. Systemic sclerosis-associated interstitial lung disease: lessons from clinical trials, outcome measures, and future study design. Curr. Rheumatol. Rev. 6, 138–144 (2010).
Christmann, R. B. et al. Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 66, 714–725 (2014).
Luzina, I. G. et al. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am. J. Respir. Cell. Mol. Biol. 26, 549–557 (2002).
Schupp, J. et al. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur. Respir. J. 43, 1530–1532 (2014).
Taroni, J. N. et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 9, 27 (2017).
Larson-Casey, J. L., Deshane, J. S., Ryan, A. J., Thannickal, V. J. & Carter, A. B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44, 582–596 (2016).
Murray, L. A. et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int. J. Biochem. Cell Biol. 40, 2174–2182 (2008).
Ishida, Y. et al. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci. Rep. 7, 16833 (2017).
Ayaub, E. A. et al. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci. Rep. 7, 1328 (2017).
Huang, J. et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 75, 883–890 (2016).
Huang, J. et al. Nintedanib macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 76, 1941–1948 (2017).
Spina, D. PDE4 inhibitors: current status. Br. J. Pharmacol. 155, 308–315 (2008).
Essayan, D. M. Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem. Pharmacol. 57, 965–973 (1999).
Maier, C. et al. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann. Rheum. Dis. 76, 1133–1141 (2017).
Schneider, D. J. et al. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J. 26, 503–512 (2012).
Nagahara, H. et al. Allograft inflammatory factor-1 in the pathogenesis of bleomycin-induced acute lung injury. Biosci. Trends 10, 47–53 (2016).
Nagahara, H. et al. Role of allograft inflammatory factor-1 in bleomycin-induced lung fibrosis. Biochem. Biophys. Res. Commun. 495, 1901–1907 (2018).
Christmann, R. B. et al. miR-155 in the progression of lung fibrosis in systemic sclerosis. Arthritis Res. Ther. 18, 155 (2016).
Gunawan, M., Jardine, L. & Haniffa, M. Isolation of human skin dendritic cell subsets. Methods Mol. Biol. 1423, 119–128 (2016).
Hall, J. C. & Rosen, A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 6, 40–49 (2010).
Ueno, H., Schmitt, N., Palucka, A. K. & Banchereau, J. Dendritic cells and humoral immunity in humans. Immunol. Cell Biol. 88, 376–380 (2010).
Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 13, 566–577 (2013).
Boltjes, A. & van Wijk, F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 5, 131 (2014).
Poulin, L. F. et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J. Exp. Med. 207, 1261–1271 (2010).
Lauterbach, H. et al. Mouse CD8alpha+ DCs and human BDCA3+DCs are major producers of IFN-lambda in response to poly IC. J. Exp. Med. 207, 2703–2717 (2010).
Chia, J. J. et al. Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. J. Clin. Invest. 126, 4331–4345 (2016).
Fleischmajer, R., Damiano, V. & Nedwich, A. Scleroderma and the subcutaneous tissue. Science 171, 1019–1021 (1971).
Siegal, F. P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999).
Guiducci, C. et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 207, 2931–2942 (2010).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Ah Kioon, M. D. et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci. Transl Med. 10, eaam8458 (2018).
Cipriani, P. et al. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic implications. Arthritis Rheum. 54, 3022–3033 (2006).
Zabel, B. A., Silverio, A. M. & Butcher, E. C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 174, 244–251 (2005).
Akamata, K. et al. Increased expression of chemerin in endothelial cells due to Fli1 deficiency may contribute to the development of digital ulcers in systemic sclerosis. Rheumatology 54, 1308–1316 (2015).
van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014).
Duan, H. et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 58, 1465–1474 (2008).
Rossato, M. et al. Association of MicroRNA-618 expression with altered frequency and activation of plasmacytoid dendritic cells in patients with systemic sclerosis. Arthritis Rheumatol. 69, 1891–1902 (2017).
Takahashi, T. et al. A potential contribution of antimicrobial peptide LL-37 to tissue fibrosis and vasculopathy in systemic sclerosis. Br. J. Dermatol. 175, 1195–1203 (2016).
Kafaja, S. et al. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight 3, 98380 (2018).
McKenna, K., Beignon, A. S. & Bhardwaj, N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79, 17–27 (2005).
Yanaba, K., Yoshizaki, A., Asano, Y., Kadono, T. & Sato, S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. J. Rheumatol. 38, 2193–2197 (2011).
Lafyatis, R. Transforming growth factor beta — at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 10, 706–719 (2014).
Usategui, A. et al. A profibrotic role for thymic stromal lymphopoietin in systemic sclerosis. Ann. Rheum. Dis. 72, 2018–2023 (2013).
Guggino, G. et al. Interleukin-9 over-expression and T helper 9 polarization in systemic sclerosis patients. Clin. Exp. Immunol. 190, 208–216 (2017).
Chakraborty, K., Chatterjee, S. & Bhattacharyya, A. Modulation of CD11c + lung dendritic cells in respect to TGF-beta in experimental pulmonary fibrosis. Cell Biol. Int. 41, 991–1000 (2017).
Gerber, E. E. et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 503, 126–130 (2013).
Siracusa, L. D. et al. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 6, 300–313 (1996).
Varghese, M. V. et al. Oxidative stress induced by the chemotherapeutic agent arsenic trioxide. 3 Biotech. 4, 425–430 (2014).
Kavian, N. et al. Arsenic trioxide prevents murine sclerodermatous graft-versus-host disease. J. Immunol. 188, 5142–5149 (2012).
Delaney, T. A. et al. Type I IFNs regulate inflammation, vasculopathy, and fibrosis in chronic cutaneous graft-versus-host disease. J. Immunol. 197, 42–50 (2016).
Municio, C. et al. Methotrexate limits inflammation through an A20-dependent cross-tolerance mechanism. Ann. Rheum. Dis. 77, 752–759 (2018).
Manno, R. & Boin, F. Immunotherapy of systemic sclerosis. Immunotherapy 2, 863–878 (2010).
Fraticelli, P. et al. Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res. Ther. 16, R144 (2014).
Kowal-Bielecka, O. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 76, 1327–1339 (2017).
Smith, V. et al. Systemic sclerosis: state of the art on clinical practice guidelines. RMD Open 4, e000782 (2018).
van Rhijn-Brouwer, F. C. C., Spierings, J. & van Laar, J. M. Autologous hematopoietic stem cell transplantation in systemic sclerosis: a reset to tolerance? Immunol. Lett. 195, 88–96 (2018).
van Laar, J. M., Naraghi, K. & Tyndall, A. Haematopoietic stem cell transplantation for poor-prognosis systemic sclerosis. Rheumatology 54, 2126–2133 (2015).
Sullivan, K. M., Shah, A., Sarantopoulos, S. & Furst, D. E. Review: hematopoietic stem cell transplantation for scleroderma: effective immunomodulatory therapy for patients with pulmonary involvement. Arthritis Rheumatol. 68, 2361–2371 (2016).
Tsukamoto, H. et al. Analysis of immune reconstitution after autologous CD34+stem/progenitor cell transplantation for systemic sclerosis: predominant reconstitution of Th1 CD4+ T cells. Rheumatology 50, 944–952 (2011).
Michel, L. et al. Evolution of serum cytokine profile after hematopoietic stem cell transplantation in systemic sclerosis patients. Bone Marrow Transplant. 51, 1146–1149 (2016).
Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125, 2795–2807 (2015).
Distler, O. et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SENSCIS). Clin. Exp. Rheumatol. 35 (Suppl. 106), 75–81 (2017).
Huang, J. et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 76, 1941–1948 (2017).
Kania, G. et al. Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ. Res. 105, 462–470 (2009).
Grimminger, F. et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur. Respir. J. 33, 785–792 (2009).
Beyer, C. et al. Stimulation of soluble guanylate cyclase reduces experimental dermal fibrosis. Ann. Rheum. Dis. 71, 1019–1026 (2012).
Beyer, C. et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFbeta signalling. Ann. Rheum. Dis. 74, 1408–1416 (2015).
Dees, C. et al. Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann. Rheum. Dis. 74, 1621–1625 (2015).
Shaw, C. A., Webb, D. J., Rossi, A. G. & Megson, I. L. Cyclic GMP protects human macrophages against peroxynitrite-induced apoptosis. J. Inflamm. 6, 14 (2009).
Mitani, H. et al. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br. J. Haematol. 109, 288–295 (2000).
Howlett, A. C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 68–69, 619–631 (2002).
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C. & Bonner, T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 (1990).
Pacher, P. & Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 50, 193–211 (2011).
Taylor, L. et al. Primary macrophage chemotaxis induced by cannabinoid receptor 2 agonists occurs independently of the CB2 receptor. Sci. Rep. 5, 10682 (2015).
Marchalant, Y., Cerbai, F., Brothers, H. M. & Wenk, G. L. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol. Aging 29, 1894–1901 (2008).
Burstein, S. H. Ajulemic acid: potential treatment for chronic inflammation. Pharmacol. Res. Perspect. 6, e00394 (2018).
Jin, Z. et al. Single-cell gene expression patterns in lupus monocytes independently indicate disease activity, interferon and therapy. Lupus Sci. Med. 4, e000202 (2017).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Krieg, C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 24, 144–153 (2018).
Blyszczuk, P., Behnke, S., Luscher, T. F., Eriksson, U. & Kania, G. GM-CSF promotes inflammatory dendritic cell formation but does not contribute to disease progression in experimental autoimmune myocarditis. Biochim. Biophys. Acta 1833, 934–944 (2013).
Blyszczuk, P. et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ. Res. 105, 912–920 (2009).
Kania, G., Blyszczuk, P. & Eriksson, U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc. Med. 19, 247–252 (2009).
Nie, Y. et al. AKT2 regulates pulmonary inflammation and fibrosis via modulating macrophage activation. J. Immunol. 198, 4470–4480 (2017).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kania, G., Rudnik, M. & Distler, O. Involvement of the myeloid cell compartment in fibrogenesis and systemic sclerosis. Nat Rev Rheumatol 15, 288–302 (2019). https://doi.org/10.1038/s41584-019-0212-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0212-z
This article is cited by
-
Nintedanib downregulates the profibrotic M2 phenotype in cultured monocyte-derived macrophages obtained from systemic sclerosis patients affected by interstitial lung disease
Arthritis Research & Therapy (2024)
-
Microparticles: potential new contributors to the pathogenesis of systemic sclerosis?
Advances in Rheumatology (2023)
-
An open-label study to evaluate biomarkers and safety in systemic sclerosis patients treated with paquinimod
Arthritis Research & Therapy (2021)
-
Serum IGFBP-2 in systemic sclerosis as a prognostic factor of lung dysfunction
Scientific Reports (2021)
-
Current Concepts on the Pathogenesis of Systemic Sclerosis
Clinical Reviews in Allergy & Immunology (2021)