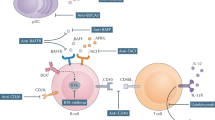
Abstract
Systemic sclerosis (SSc) has the highest cause-specific mortality of all the connective tissue diseases, and the aetiology of this complex and heterogeneous condition remains an enigma. Current disease-modifying therapies for SSc predominantly target inflammatory and vascular pathways but have variable and unpredictable clinical efficacy, and none is curative. Moreover, many of these therapies possess undesirable safety profiles and have no appreciable effect on long-term mortality. This Review describes the most promising of the existing therapeutic targets for SSc and places them in the context of our evolving understanding of the pathophysiology of this disease. As well as taking an in-depth look at the immune, inflammatory, vascular and fibrotic pathways implicated in the pathogenesis of SSc, this Review discusses emerging treatment targets and therapeutic strategies. The article concludes with an overview of important unanswered questions in SSc research that might inform the design of future studies of treatments aimed at modifying the course of this disease.
Key points
-
Systemic sclerosis (SSc) is associated with the highest mortality among the rheumatic diseases and to date lacks approved disease-modifying therapies.
-
The clinical presentation and course of SSc are highly variable, which reflects interpatient genetic and molecular heterogeneity.
-
Advances in our understanding of the immune, vascular and fibrotic mechanisms underlying the pathogenesis of SSc have revealed a growing number of potential therapeutic targets.
-
Clinical trials have shown some efficacy of therapies for SSc that target immune cell types and mediators and cellular pathways that drive fibrosis.
-
Key challenges in the development of effective disease-modifying therapies for SSc include patient heterogeneity and a lack of consensus on optimal trial design and efficacy end points.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Primers 1, 15002 (2015).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Varga, J., Trojanowska, M. & Kuwana, M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2, 137–152 (2017).
Townsend, M. J. & Arron, J. R. Reducing the risk of failure: biomarker-guided trial design. Nat. Rev. Drug Discov. 15, 517–518 (2016).
Asmani, M. et al. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat. Commun. 9, 2066 (2018).
Kowal-Bielecka, O. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 76, 1327–1339 (2017).
Tashkin, D. P. et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 4, 708–719 (2016).
Fernández-Codina, A., Walker, K. M. & Pope, J. E. Scleroderma Algorithm Group. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol. 70, 1820–1828 (2018).
Varga, J. & Abraham, D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 (2007).
Bhattacharyya, S., Wei, J. & Varga, J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 8, 42–54 (2011).
Hinz, B. et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355 (2012).
Pakshir, P. & Hinz, B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 68–69, 81–93 (2018).
Santos, A. & Lagares, D. Matrix stiffness: the conductor of organ fibrosis. Curr. Rheumatol. Rep. 20, 2 (2018).
Varga, J. & Pasche, B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat. Rev. Rheumatol. 5, 200–206 (2009).
Lafyatis, R. Transforming growth factor β — at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 10, 706–719 (2014).
Kim, K. K., Sheppard, D. & Chapman, H. A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 10, a022293 (2018).
Sargent, J. L. et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J. Invest. Dermatol. 130, 694–705 (2010).
Robertson, I. B. & Rifkin, D. B. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb. Perspect. Biol. 8, a021907 (2016).
Xiao, H. et al. Metformin is a novel suppressor for transforming growth factor (TGF)-β1. Sci. Rep. 6, 28597 (2016).
Tsou, P. S., Haak, A. J., Khanna, D. & Neubig, R. R. Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription. Am. J. Physiol. Cell Physiol. 307, C2–C13 (2014).
Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125, 2795–2807 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01371305 (2018).
Bhattacharyya, S. et al. A non-SMAD mechanism of fibroblast activation by transforming growth factor-β via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 28, 1285–1297 (2009).
Akhmetshina, A. et al. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 60, 219–224 (2009).
Spiera, R. F. et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Ann. Rheum. Dis. 70, 1003–1009 (2011).
Pope, J. et al. Imatinib in active diffuse cutaneous systemic sclerosis: results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 63, 3547–3551 (2011).
Martyanov, V. et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLOS ONE 12, e0187580 (2017).
Gordon, J. K. et al. Nilotinib (Tasigna™) in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial. Arthritis Res. Ther. 17, 213 (2015).
Huang, J. et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 76, 1941–1948 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02597933 (2018).
Distler, J. H. et al. Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis Rheumatol. 69, 257–267 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03041025 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01890265 (2018).
Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 174, 519–533 (2009).
Wei, J. et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLOS ONE 5, e13778 (2010).
Marangoni, R. G. et al. A candidate gene study reveals association between a variant of the peroxisome proliferator-activated receptor gamma (PPAR-γ) gene and systemic sclerosis. Arthritis Res. Ther. 17, 128 (2015).
Avouac, J. et al. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Ann. Rheum. Dis. 76, 1931–1940 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02503644 (2018).
Bogatkevich, G. S. et al. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 63, 1416–1425 (2011).
Shea, B. S. et al. Uncoupling the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2, 86608 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02426229 (2018).
Spadoni, T. et al. A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis. Arthritis Rheumatol. 67, 1611–1622 (2015).
Piera-Velazquez, S., Makul, A. & Jiménez, S. A. Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β. Arthritis Rheumatol. 67, 2749–2758 (2015).
Nanthakumar, C. B. et al. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov. 14, 693–720 (2015).
Wei, J. et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces SMAD-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 64, 2734–2745 (2012).
Beyer, C. et al. Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann. Rheum. Dis. 72, 1255–1258 (2013).
Lafyatis, R. et al. Inhibition of β-catenin signaling in the skin rescues cutaneous adipogenesis in systemic sclerosis: a randomized, double-blind, placebo-controlled trial of C-82. J. Invest. Dermatol. 137, 2473–2483 (2017).
Horn, A. et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 64, 2724–2733 (2012).
Didiasova, M. et al. Pirfenidone exerts antifibrotic effects through inhibition of GLI transcription factors. FASEB J. 31, 1916–1928 (2017).
Xiao, H. et al. Anti-fibrotic effects of pirfenidone by interference with the Hedgehog signaling pathway in patients with systemic sclerosis-associated interstitial lung disease. Int. J. Rheum. Dis. 21, 477–486 (2018).
Khanna, D. et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS Trial. J. Rheumatol. 43, 1672–1679 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03221257 (2018).
Desallais, L. et al. Targeting IL-6 by both passive or active immunization strategies prevents bleomycin-induced skin fibrosis. Arthritis Res. Ther. 16, R157 (2014).
Kitaba, S. et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am. J. Pathol. 180, 165–176 (2012).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum. Dis. 77, 212–220 (2018).
Denton, C. P. et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-β pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann. Rheum. Dis. 77, 1362–1371 (2018).
Fuschiotti, P. Role of IL-13 in systemic sclerosis. Cytokine 56, 544–549 (2011).
Allanore, Y. et al. Double-blind, randomized, 8-week placebo-controlled followed by a 16-week open label extension study, with the LPA1 receptor antagonist SAR100842 for patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 70, 1634–1643 (2018).
Zurier, R. B. & Burstein, S. H. Cannabinoids, inflammation, and fibrosis. FASEB J. 30, 3682–3689 (2016).
Gonzalez, E. G. et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann. Rheum. Dis. 71, 1545–1551 (2012).
del Río, C. et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci. Rep. 6, 21703 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02465437 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03398837 (2019).
Katz-Talmor, D. et al. Cannabinoids for the treatment of rheumatic diseases — where do we stand? Nat. Rev. Rheumatol. 14, 488–498 (2018).
O’Reilly, S. Toll like receptors in systemic sclerosis: an emerging target. Immunol. Lett. 195, 2–8 (2018).
Bhattacharyya, S. et al. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 182, 192–205 (2013).
Bhattacharyya, S. & Varga, J. Endogenous ligands of TLR4 promote unresolving tissue fibrosis: implications for systemic sclerosis and its targeted therapy. Immunol. Lett. 195, 9–17 (2018).
Tomcik, M. et al. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-β signalling to prevent fibrosis. Ann. Rheum. Dis. 73, 1215–1222 (2014).
Takahashi, T. et al. Amelioration of tissue fibrosis by Toll-like receptor 4 knockout in murine models of systemic sclerosis. Arthritis Rheumatol. 67, 254–265 (2015).
Christmann, R. B. et al. Association of interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 66, 714–725 (2014).
Trujillo, G. et al. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci. Transl Med. 2, 57ra82 (2010).
Kirillov, V. et al. Sustained activation of Toll-like receptor 9 induces an invasive phenotype in lung fibroblasts: possible implications in idiopathic pulmonary fibrosis. Am. J. Pathol. 185, 943–957 (2015).
Fang, F. et al. Toll-like receptor 9 signaling is augmented in systemic sclerosis and elicits transforming growth factor β-dependent fibroblast activation. Arthritis Rheumatol. 68, 1989–2002 (2016).
Gheita, T. A. et al. Toll-like receptor 9 in systemic sclerosis patients: relation to modified Rodnan skin score, disease severity, and functional status. Clin. Rheumatol. 37, 757–763 (2018).
Affandi, A. J., Carvalheiro, T., Radstake, T. R. D. J. & Marut, W. Dendritic cells in systemic sclerosis: advances from human and mice studies. Immunol. Lett. 195, 18–29 (2018).
Van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014).
Divekar, A. A. et al. pDCs and IL-4+ T cells in scleroderma as novel targets of imatinib mesylate. J. Immunol. 186, 44 (2011).
Kafaja, S. et al. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight 3, e98380 (2018).
Rossato, M. et al. Association of microRNA-618 expression with altered frequency and activation of plasmacytoid dendritic cells in patients with systemic sclerosis. Arthritis Rheumatol. 69, 1891–1902 (2017).
Peng, W. J. MicroRNA-29: a potential therapeutic target for systemic sclerosis. Expert Opin. Ther. Targets 16, 875–879 (2012).
Wynn, T. A. & Vanella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02550873 (2018).
King, J., Abraham, D. & Stratton, R. Chemokines in systemic sclerosis. Immunol. Lett. 195, 68–75 (2018).
Castelino, F. V. & Varga, J. Current status of systemic sclerosis biomarkers: applications for diagnosis, management and drug development. Expert Rev. Clin. Immunol. 9, 1077–1090 (2013).
Wermuth, P., Piera-Velazquez, S., Rosenbloom, J. & Jimenez, S. A. Existing and novel biomarkers for precision medicine in systemic sclerosis. Nat. Rev. Rheumatol. 14, 421–432 (2018).
Ferreira, A. M. et al. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Invest. Dermatol. 126, 1900–1908 (2006).
Distler, O. et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 44, 2665–2678 (2001).
Antonelli, A. et al. CXCL10 (α) and CCL2 (β) chemokines in systemic sclerosis — a longitudinal study. Rheumatology 47, 45–49 (2008).
Carulli, M. T. et al. Can CCL2 serum levels be used in risk stratification or to monitor treatment response in systemic sclerosis? Ann. Rheum. Dis. 67, 105–109 (2008).
Wu, M. et al. CCL2 in the circulation predicts long-term progression of interstitial lung disease in patients with early systemic sclerosis: data from two independent cohorts. Arthritis Rheumatol. 69, 1871–1878 (2017).
Liu, X. et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum. 65, 226–235 (2013).
Volkmann, E. R. et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res. Ther. 18, 305 (2016).
Puengel, T. et al. Differential impact of the dual CCR2/CCR5 inhibitor cenicriviroc on migration of monocyte and lymphocyte subsets in acute liver injury. PLOS ONE 12, e0184694 (2017).
Haringman, J. J. et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 54, 2387–2392 (2006).
Vergunst, C. E. et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939 (2008).
Tak, P. P. et al. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. Ann. Rheum. Dis. 72, 337–344 (2013).
Gadina, M. et al. Translational and clinical advances in JAK–STAT biology: the present and future of jakinibs. J. Leukoc. Biol. 104, 499–514 (2018).
Villarino, A. V., Kanno, Y. & O’Shea, J. J. Mechanisms and consequences of JAK–STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017).
Muskardin, T. L. W. & Niewold, T. B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 14, 214–228 (2018).
Costa, L. et al. Small molecule therapy for managing moderate to severe psoriatic arthritis. Expert Opin. Pharmacother. 18, 1557–1567 (2017).
Dubash, S., McGonagle, D. & Marzo-Ortega, H. New advances in the understanding and treatment of axial spondyloarthritis: from chance to choice. Ther. Adv. Chronic Dis. 9, 77–87 (2018).
White, J. R. et al. Review article: novel oral-targeted therapies in inflammatory bowel disease. Aliment. Pharmacol. Ther. 47, 1610–1622 (2018).
Kahn, J. S., Deverapalli, S. C. & Rosmarin, D. M. JAK–STAT signaling pathway inhibition: a role for treatment of discoid lupus erythematosus and dermatomyositis. Int. J. Dermatol. 57, 1007–1014 (2018).
Dees, C. et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum. 64, 3006–3015 (2012).
Chakraborty, D., Šumová, B. & Mallano, T. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 8, 1130 (2017).
Xu, Y. et al. Polymorphisms in STAT4 and IRF5 increase the risk of systemic sclerosis: a meta-analysis. Int. J. Dermatol. 55, 408–416 (2016).
Dieudé, P. et al. STAT4 is a genetic risk factor for systemic sclerosis having additive effects with IRF5 on disease susceptibility and related pulmonary fibrosis. Arthritis Rheum. 60, 2472–2479 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03274076 (2018).
Deverapalli, S. C. & Rosmarin, D. The use of JAK inhibitors in the treatment of progressive systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 32, e328 (2018).
Sobanski, V. et al. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol. 66, 407–417 (2014).
Kraaij, M. D. & van Laar, J. M. The role of B cells in systemic sclerosis. Biologics 2, 389–395 (2008).
Manetti, M. et al. Endothelial/lymphocyte activation leads to prominent CD4+T cell infiltration in the gastric mucosa of patients with systemic sclerosis. Arthritis Rheum. 58, 2866–2873 (2008).
Lafyatis, R., O’Hara, C., Feghali-Bostwick, C. A. & Matteson, E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 56, 3167–3168 (2007).
De Santis, M. et al. Bronchoalveolar lavage fluid and progression of scleroderma interstitial lung disease. Clin. Respir. J. 6, 9–17 (2012).
Jordan, S. et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann. Rheum. Dis. 74, 1188–1194 (2015).
Sato, S., Fujimoto, M., Hasegawa, M. & Takehara, K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 50, 1918–1927 (2004).
Gambichler, T. et al. Absolute count of T and B lymphocyte subsets is decreased in systemic sclerosis. Eur. J. Med. Res. 15, 44–46 (2010).
Sanges, S. et al. Role of B cells in the pathogenesis of systemic sclerosis. Rev. Med. Interne 38, 113–124 (2017).
Mavropoulos, A. et al. Breg cells are numerically decreased and functionally impaired in patients with systemic sclerosis. Arthritis Rheumatol. 68, 494–504 (2016).
Matsushita, T. et al. Decreased levels of regulatory B cells in patients with systemic sclerosis: association with autoantibody production and disease activity. Rheumatology 55, 263–267 (2016).
Asano, N. et al. B Lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight-skin mouse. Am. J. Pathol. 165, 641–650 (2004).
Soto, L. et al. Systemic sclerosis patients present alterations in the expression of molecules involved in B cell regulation. Front. Immunol. 6, 496 (2015).
Sato, S. et al. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J. Immunol. 165, 6635–6643 (2000).
Brkic, Z. et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 75, 1567–1573 (2016).
Matsushita, T. et al. Elevated serum APRIL levels in patients with systemic sclerosis: distinct profiles of systemic sclerosis categorized by APRIL and BAFF. J. Rheumatol. 34, 2056–2062 (2007).
Bielecki, M. et al. Increased production of a proliferation-inducing ligand (APRIL) by peripheral blood mononuclear cells is associated with anti-topoisomerase I antibody and more severe disease in systemic sclerosis. J. Rheumatol. 37, 2286–2289 (2010).
Nardelli, B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001).
Matsushita, T. et al. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 54, 192–201 (2006).
Matsushita, T. et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci. Adv. 4, eaas9944 (2018).
Bassyouni, H. et al. Elevated serum levels of a proliferation-inducing ligand in patients with systemic sclerosis: possible association with myositis? Joint Bone Spine 78, 56–61 (2011).
Thiebaut, M. et al. Efficacy and safety of rituximab in systemic sclerosis: French retrospective study and literature review. Autoimmun. Rev. 17, 582–587 (2018).
Daoussis, D. et al. A multicenter, open-label, comparative study of B cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Semin. Arthritis Rheum. 46, 625–631 (2017).
Streicher, K. et al. Baseline plasma cell gene signature predicts improvement in systemic sclerosis skin scores following treatment with inebilizumab (MEDI-551) and correlates with disease activity in systemic lupus erythematosus and chronic obstructive pulmonary disease. Arthritis Rheumatol. 70, 2087–2095 (2018).
Gordon, J. K. et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol. 70, 308–316 (2018).
Brembilla, N. C. & Chizzolini, C. T cell abnormalities in systemic sclerosis with a focus on TH17 cells. Eur. Cytokine Netw. 23, 128–139 (2012).
O’Reilly, S., Hugle, T. & van Laar, J. M. T cells in systemic sclerosis: a reappraisal. Rheumatology 51, 1540–1549 (2012).
Truchetet, M. E. Increased frequency of circulating TH22 in addition to TH17 and TH2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res. Ther. 13, R166 (2011).
Rodriguez-Reyna, T. S. et al. TH17 peripheral cells are increased in diffuse cutaneous systemic sclerosis compared with limited illness: a cross-sectional study. Rheumatol. Int. 32, 2653–2660 (2012).
Liston, A. & Gray, D. H. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 14, 154–165 (2014).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
MacDonald, K. G. et al. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J. Allergy Clin. Immunol. 135, 946 (2015).
Slobodin, G. & Rimar, D. Regulatory T cells in systemic sclerosis: a comprehensive review. Clin. Rev. Allergy Immunol. 52, 194–201 (2017).
Slobodin, G. et al. Regulatory T cells (CD4+CD25brightFoxP3+) expansion in systemic sclerosis correlates with disease activity and severity. Cell. Immunol. 261, 77–80 (2010).
Jiang, N., Li, M. & Zeng, X. Correlation of TH17 cells and CD4+CD25+ regulatory T cells with clinical parameters in patients with systemic sclerosis. Chin. Med. J. 127, 3557–3561 (2014).
Becker, M. O. et al. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: an open-label study. Ann. Rheum. Dis. 70, 1340–1341 (2011).
Ponsoye, M. et al. Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation-driven fibrosis. Ann. Rheum. Dis. 75, 2142–2149 (2016).
Elhai, M. et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann. Rheum. Dis. 72, 1217–1220 (2013).
Khanna, D. et al. Abatacept versus placebo in early diffuse cutaneous systemic sclerosis — results of a phase 2 investigator initiated, double-blind, placebo-controlled, multicenter, randomized controlled trial. Arthritis Rheumatol. 70 (Suppl. 10), 900 (2018).
van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation versus intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311, 2490–2498 (2014).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Sullivan, K. M. et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N. Engl. J. Med. 378, 35–47 (2018).
van Rhijn-Brouwer, F. C. et al. Cellular therapies in systemic sclerosis: recent progress. Curr. Rheumatol. Rep. 18, 12 (2016).
Sullivan, K. M. et al. Systemic sclerosis as an indication for autologous hematopoietic cell transplantation: position statement from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 24, 1961–1964 (2018).
Arruda, L. C. M. et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv. 2, 126–141 (2018).
Baraut, J. et al. Peripheral blood regulatory T cells in patients with diffuse systemic sclerosis (SSc) before and after autologous hematopoietic SCT: a pilot study. Bone Marrow Transplant. 49, 349–354 (2014).
Szodoray, P. et al. Immunological reconstitution after autologous stem cell transplantation in patients with refractory systemic autoimmune diseases. Scand. J. Rheumatol. 41, 110–115 (2012).
Khanna, D. et al. Adipose-derived cell therapy for hand dysfunction in patients with systemic sclerosis: a randomized, double-blind, placebo-controlled trial. J. Scleroderma Relat. Disord. 3, 69–101 (2018).
Matucci-Cerinic, M. et al. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 65, 1953–1962 (2013).
Frech, T. M. et al. Implications of endothelial shear stress on systemic sclerosis vasculopathy and treatment. Clin. Exp. Rheumatol. 36, 175–182 (2018).
Abdulle, A. E., Diercks, G. F. H., Feelisch, M., Mulder, D. J. & van Goor, H. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front. Physiol. 9, 1177 (2018).
Bruni, C. et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front. Immunol. 9, 204 (2018).
Simonneau, G. et al. Selexipag, an oral, selective IP receptor agonist for the treatment of pulmonary arterial hypertension. Eur. Respir. J. 40, 874–880 (2012).
Cutolo, M. et al. Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts. Arthritis Res. Ther. 20, 77 (2018).
Denton, C. P. et al. Efficacy and safety of selexipag in adults with Raynaud’s phenomenon secondary to systemic sclerosis: a randomized, placebo-controlled, phase II study. Arthritis Rheumatol. 69, 2370–2379 (2017).
Gaine, S. et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur. Respir. J. 50, 1602493 (2017).
Puliod, T. et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 369, 809–818 (2013).
Khanna, D. et al. Effect of macitentan on the development of new ischemic digital ulcers in patients with systemic sclerosis: DUAL-1 and DUAL-2 randomized clinical trials. JAMA 315, 1975–1988 (2016).
Kuwana, M., Okazaki, Y. & Kaburaki, J. Long-term beneficial effects of statins on vascular manifestations in patients with systemic sclerosis. Mod. Rheumatol. 19, 530–535 (2009).
Furukawa, S. et al. Protective effect of pravastatin on vascular endothelium in patients with systemic sclerosis: a pilot study. Ann. Rheum. Dis. 65, 1118–1120 (2006).
Abou-Raya, A., Abou-Raya, S. & Helmii, M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J. Rheumatol. 35, 1801–1808 (2008).
Sadik, H. Y. et al. Lack of effect of 8 weeks atorvastatin on microvascular endothelial function in patients with systemic sclerosis. Rheumatology 49, 990–996 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02370784 (2018).
Grzegorzewska, A. P. et al. Dimethyl fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci. Rep. 7, 41605 (2017).
Kastrati, I. et al. Dimethyl fumarate inhibits the nuclear factor κB pathway in breast cancer cells by covalent modification of p65 protein. J. Biol. Chem. 291, 3639–3647 (2016).
Toyama, T. et al. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J. Invest. Dermatol. 138, 78–88 (2018).
Wei, J. et al. Nrf2 exerts cell-autonomous antifibrotic effects: compromised function in systemic sclerosis and therapeutic rescue with a novel heterocyclic chalcone derivative. Transl Res. 183, 71–86 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02981082 (2018).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Tschumperlin, D. J., Ligresti, G., Hilscher, M. B. & Shah, V. H. Mechanosensing and fibrosis. J. Clin. Invest. 128, 74–84 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02745145 (2018).
Kurundkar, A. & Thannickal, V. J. Redox mechanisms in age-related lung fibrosis. Redox Biol. 9, 67–76 (2016).
Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl Med. 9, eaal3765 (2017).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Wei, J. et al. The histone deacetylas sirtuin 1 is reduced in systemic sclerosis and abrogates fibrotic responses by targeting transforming growth factor β signaling. Arthritis Rheumatol. 67, 1323–1334 (2015).
Zerr, P. et al. Sirt1 regulates canonical TGF-β signaling to control fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 75, 226–233 (2016).
Akamata, K. et al. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7, 69321–68336 (2016).
Tarrago, M. G. et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 27, 1081–1095 (2018).
Chini, E. N. et al. The pharmacology of CD38/NADase: an emerging target in cancer and diseases of aging. Trends Pharmacol. Sci. 39, 424–436 (2018).
Fang, F. et al. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Res. Ther. 14, R229 (2012).
Ursini, F. et al. Metformin and autoimmunity: a “new deal” of an old drug. Front. Immunol. 9, 1236 (2018).
Angiolilli, C. et al. New insights into the genetics and epigenetics of systemic sclerosis. Nat. Rev. Rheumatol. 14, 657–673 (2018).
Tsou, P. S. & Sawalha, A. H. Unfolding the pathogenesis of scleroderma. J. Autoimmun. 83, 73–94 (2017).
Taylor, D. K. et al. T follicular helper-like cells contribute to skin fibrosis. Sci. Transl Med. 10, eaaf5307 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01748084 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01086540 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01862926 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02161406 (2018).
Acknowledgements
The authors thank D. Legares, S. Bhattacharyya and B. Korman for helpful discussions. The authors’ research is supported by grants from the National Institutes of Health (AR42309 and R56AG054207 to J.V. and E.R.V.) and a grant from the Rheumatology Research Foundation (to E.R.V.).
Review criteria
Articles for inclusion in this narrative review and overview article were selected by searching PubMed to identify relevant articles published in the past 5 years using the following search terms: “systemic sclerosis” OR “scleroderma” and “treatment” OR “therapy”. The abstracts of retrieved articles were reviewed to determine whether they described an inflammatory target, a fibrosis target or a vascular target, and articles that described none of these were eliminated. Additionally, the ClinicalTrials.gov database was searched using the terms “scleroderma” or “systemic sclerosis” to identify relevant clinical trials published within the past 5 years.
Reviewer information
Nature Reviews Rheumatology thanks C. Buckley, M. Matucci-Cerinic, and other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
E.R.V. declares that she has acted as a clinical trial investigator for Boehringer-Ingelheim and Genentech/Roche and is an advisory board member for Boehringer-Ingelheim. J.V. declares that he has acted as a clinical trial investigator for Corbus, Cytori, GlaxoSmithKline and Genentech/Roche; has received research grants from Bristol-Myers Squibb, Pfizer and the National Institutes of Health (NIH); and is an advisory board member for Boehringer-Ingelheim, Corbus, Emerald, Inventiva and Mitsubishi.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Volkmann, E.R., Varga, J. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat Rev Rheumatol 15, 208–224 (2019). https://doi.org/10.1038/s41584-019-0184-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0184-z
This article is cited by
-
Heavy-chain antibody targeting of CD38 NAD+ hydrolase ectoenzyme to prevent fibrosis in multiple organs
Scientific Reports (2023)
-
Immune cell dysregulation as a mediator of fibrosis in systemic sclerosis
Nature Reviews Rheumatology (2022)
-
An update on targeted therapies in systemic sclerosis based on a systematic review from the last 3 years
Arthritis Research & Therapy (2021)
-
Serum IGFBP-2 in systemic sclerosis as a prognostic factor of lung dysfunction
Scientific Reports (2021)
-
Therapeutic Approaches to Systemic Sclerosis: Recent Approvals and Future Candidate Therapies
Clinical Reviews in Allergy & Immunology (2021)