Abstract
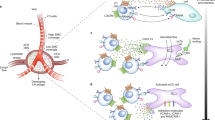
The trafficking of leukocytes from their site of production in the bone marrow through the circulation and into peripheral tissues is a highly coordinated and tightly regulated process in healthy individuals. Lymphocytes are long-lived cells that visit many lymphoid and peripheral tissues over their lifetime and can even recirculate back to the bone marrow, whereas granulocytes and monocytes are not thought to recirculate so widely. Using rheumatoid arthritis (RA) as an example, this Review explores the migratory journey of leukocytes during the establishment and resolution of disease — from the blood, through the lymphoid tissues and into peripheral sites such as the lungs and the gut before their entry into the synovium. This Review explores our current understanding of differences in the molecular processes that regulate leukocyte trafficking at different phases of disease and in different stromal compartments, which could help to explain the disease heterogeneity seen in patients with RA. Expanding our knowledge of these processes will open new avenues in the clinical management of RA, paving the way for personalized medicine that is founded on the pathological molecular signature of each patient, which varies according to their phase of disease or disease subtype.
Key points
-
Patients with rheumatoid arthritis (RA) have defects in at least one, if not multiple, checkpoints that regulate leukocyte entry into and exit from lymphoid and peripheral tissues, including the joints.
-
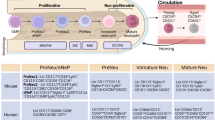
Even at the earliest phases of disease, patients with RA have impaired thymic output and naive T cells that have an immunosenescent phenotype.
-
Individuals at risk of RA show signs of alterations in the phenotype of lymphocytes trafficking through the draining lymph nodes of the joints.
-
Genetic predisposition and metabolic alterations in T cells can render them ‘sticky’ and hypermotile, contributing to aberrant lymphocyte trafficking throughout the body.
-
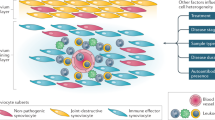
Interactions between fibroblasts and endothelial cells in the synovium evolve with disease, changing the shape and nature of the inflammatory infiltrate at each phase of RA.
-
Lymphatic vessels change during experimental inflammatory arthritis and represent a potential novel therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Doita, M., Maeda, S., Kawai, K., Hirohata, K. & Sugiyama, T. Analysis of lymphocyte subsets of bone marrow in patients with rheumatoid arthritis by two colour immunofluorescence and flow cytometry. Ann. Rheum. Dis. 49, 168–171 (1990).
Hirohata, S. et al. Accelerated generation of CD14+ monocyte-lineage cells from the bone marrow of rheumatoid arthritis patients. Arthritis Rheum. 5, 836–843 (1996).
Bugatti, S. et al. Ultrasonographic and MRI characterisation of the palindromic phase of rheumatoid arthritis. Ann. Rheum. Dis. 71, 625–626 (2012).
Jimenez-Boj, E. et al. Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J. Immunol. 175, 2579–2588 (2005).
Cosway, E., Anderson, G., Garside, P. & Prendergast, C. The thymus and rheumatology: should we care? Curr. Opin. Rheumatol. 28, 189–195 (2016).
Wagner, U., Schatz, A., Baerwald, C. & Rossol, M. Brief report: Deficient thymic output in rheumatoid arthritis despite abundance of prethymic progenitors. Arthritis Rheum. 65, 2567–2572 (2013).
McGovern, J. L. et al. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 64, 3129–3138 (2012).
Nadkarni, S., Mauri, C. & Ehrenstein, M. R. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J. Exp. Med. 204, 33–39 (2007).
Byng-Maddick, R. & Ehrenstein, M. R. The impact of biological therapy on regulatory T cells in rheumatoid arthritis. Rheumatology 54, 768–775 (2015).
Ponchel, F. et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 100, 4550–4556 (2002).
Koetz, K. et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 9203–9208 (2000).
Hale, J. S. & Fink, P. J. Back to the thymus: peripheral T cells come home. Immunol. Cell Biol. 87, 58–64 (2009).
Thiault, N. et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 16, 628–634 (2015).
Edelmann, S. L., Marconi, P. & Brocker, T. Peripheral T cells re-enter the thymus and interfere with central tolerance induction. J. Immunol. 186, 5612–5619 (2011).
Frommer, F. & Waisman, A. B cells participate in thymic negative selection of murine auto-reactive CD4(+) T cells. PLoS ONE 5, e15372 (2010).
Hodge, D. L. et al. MCP-1/CCR2 interactions direct migration of peripheral B and T lymphocytes to the thymus during acute infectious/inflammatory processes. Eur. J. Immunol. 42, 2644–2654 (2012).
Cowan, J., McCarthy, N. & Anderson, G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3(+) T cells. Cell Rep. 14, 1041–1048 (2016).
Tracy, A., Buckley, C. D. & Raza, K. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Semin. Immunopathol. 39, 423–435 (2017).
Stolt, P. et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann. Rheum. Dis. 62, 835–841 (2003).
Izquierdo, E. et al. Immature blood vessels in rheumatoid synovium are selectively depleted in response to anti-TNF therapy. PLoS ONE 4, e8131 (2009).
Potempa, J., Mydel, P. & Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 606–620 (2017).
Queiroz-Junior, C. M. et al. Experimental arthritis triggers periodontal disease in mice: involvement of TNF-α and the oral microbiota. J. Immunol. 187, 3821–3830 (2011).
Queiroz-Junior, C. M. et al. Experimental arthritis exacerbates Aggregatibacter actinomycetemcomitans-induced periodontitis in mice. J. Clin. Periodontol. 39, 608–616 (2012).
Ramwadhdoebe, T. H. et al. Human lymph-node CD8(+) T cells display an altered phenotype during systemic autoimmunity. Clin. Transl Immunol. 5, e67 (2016).
Ramwadhdoebe, T. H. et al. Lymph node biopsy analysis reveals an altered immunoregulatory balance already during the at-risk phase of autoantibody positive rheumatoid arthritis. Eur. J. Immunol. 46, 2812–2821 (2016).
Hañhnlein, J. S. et al. Distinctive expression of T cell guiding molecules in human autoimmune lymph node stromal cells upon TLR3 triggering. Sci. Rep. 8, 1736 (2018).
Hähnlein, J. S. et al. Impaired lymph node stromal cell function during the earliest phases of rheumatoid arthritis. Arthritis Res. Ther. 20, 35 (2018).
Li, J. et al. Expanded CD23/CD21 B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. J. Immunol. 184, 6142–6150 (2010).
Rodríguez-Palmero, M. et al. Alterations of lymphocyte populations in lymph nodes but not in spleen during the latency period of adjuvant arthritis. Inflammation 23, 153–165 (1999).
Nourshargh, S. & Alon, R. Leukocyte migration into inflamed tissues. Immunity 41, 694–707 (2014).
Mellado, M. et al. T cell migration in rheumatoid arthritis. Front. Immunol. 6, 384 (2015).
Luo, D., McGettrick, H. M., Stone, P. C., Rainger, G. E. & Nash, G. B. The roles of integrins in function of human neutrophils after their migration through endothelium into interstitial matrix. PLoS ONE 10, e0118593 (2015).
McGettrick, H. M. et al. Chemokine- and adhesion-dependent survival of neutrophils after transmigration through cytokine-stimulated endothelium. J. Leukoc. Biol. 79, 779–788 (2006).
Filer, A. et al. Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils. Arthritis Rheum. 54, 2096–2108 (2006).
Bayley, R. et al. The autoimmune-associated genetic variant PTPN22 R620W enhances neutrophil activation and function in patients with rheumatoid arthritis and healthy individuals. Ann. Rheum. Dis. 74, 1588–1595 (2015).
Burn, G. L. et al. Superresolution imaging of the cytoplasmic phosphatase PTPN22 links integrin-mediated T cell adhesion with autoimmunity. Sci. Signal. 9, ra99 (2016).
Hwang, S. H. et al. Leukocyte-specific protein 1 regulates T cell migration in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 112, E6535–E6543 (2015).
Shen, Y. et al. Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat. Immunol. 18, 1025–1034 (2017).
Demoruelle, M. K. et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 69, 1165–1175 (2017).
McComb, J. G. et al. CX3CL1 up-regulation is associated with recruitment of CX3CR1(+) mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. Am. J. Pathol. 173, 949–961 (2008).
Qiu, C. et al. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunology 138, 76–82 (2013).
Qiu, C. et al. Hydrodynamic delivery of IL-28B (IFN-λ3) gene ameliorates lung inflammation induced by cigarette smoke exposure in mice. Biochem. Biophys. Res. Commun. 447, 513–519 (2014).
Nowak, D., Ruta, U. & Piasecka, G. Nicotine increases human polymorphonuclear leukocytes chemotactic response - a possible additional mechanism of lung injury in cigarette smokers. Exp. Pathol. 39, 37–43 (1990).
Ryder, M. I. et al. Alterations of neutrophil L-selection and CD18 expression by tobacco smoke: implications for periodontal diseases. J. Periodont. Res. 33, 359–368 (1998).
Stone, P. C. W., Fisher, A. C., Rainger, G. E. & Nash, G. B. Neutrophil capture by selectins on endothelial cells exposed to cigarette smoke. Biochem. Biophys. Res. Commun. 295, 1150–1155 (2002).
Overbeek, S. A. et al. Cigarette smoke induces β2-integrin-dependent neutrophil migration across human endothelium. Respir. Res. 12, 75–75 (2011).
Laan, M., Bozinovski, S. & Anderson, G. P. Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J. Immunol. 173, 4164–4170 (2004).
Vassallo, R. et al. Cellular and humoral immunity in arthritis are profoundly influenced by the interaction between cigarette smoke effects and host HLA-DR and DQ genes. Clin. Immunol. 152, 25–35 (2014).
Allais, L. et al. The effect of cigarette smoke exposure on the development of inflammation in lungs, gut and joints of TNFΔARE mice. PLoS ONE 10, e0141570 (2015).
Glossop, J. R., Dawes, P. T. & Mattey, D. L. Association between cigarette smoking and release of tumour necrosis factor-α and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology 45, 1223–1229 (2006).
Reynisdottir, G. et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann. Rheum. Dis. 75, 1722–1727 (2016).
Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905 (2015).
Scher, J. U. et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 4, 60 (2016).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Morton, A. M. et al. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc. Natl Acad. Sci. USA 111, 6696–6701 (2014).
Naskar, D., Teng, F., Felix, K. M., Bradley, C. P. & Wu, H. J. J. Synthetic retinoid AM80 ameliorates lung and arthritic autoimmune responses by inhibiting T follicular helper and Th17 cell responses. J. Immunol. 198, 1855–1864 (2017).
Block, K. E., Zheng, Z., Dent, A. L., Kee, B. L. & Huang, H. Gut microbiota regulates K/B×N autoimmune arthritis through Tfh but not Th17 cells. J. Immunol. 196, 1550–1557 (2016).
Teng, F. et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer's patch T follicular helper cells. Immunity 44, 875–888 (2016).
Chappert, P., Bouladoux, N., Naik, S. & Schwartz, R. H. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity 38, 1198–1210 (2013).
Salmi, M., Rajala, P. & Jalkanen, S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J. Clin. Invest. 99, 2165–2172 (1997).
Tong, B. et al. Sinomenine suppresses collagen-induced arthritis by reciprocal modulation of regulatory T cells and Th17 cells in gut-associated lymphoid tissues. Mol. Immunol. 65, 94–103 (2015).
Yue, M. et al. Berberine ameliorates collagen-induced arthritis in rats by suppressing Th17 cell responses via inducing cortistatin in the gut. FEBS J. 284, 2786–2801 (2017).
Marietta, E. V. et al. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol. 68, 2878–2888 (2016).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Kaufman, A. & Herold, K. C. Anti-CD3 mAbs for treatment of type 1 diabetes. Diabetes Metab. Res. Rev. 25, 302–306 (2009).
Emery, P. et al. Impact of T cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann. Rheum. Dis. 69, 510–516 (2010).
Maxwell, L. J. & Singh, J. A. Abatacept for rheumatoid arthritis: a Cochrane systematic review. J. Rheumatol. 37, 234–245 (2010).
Asquith, D. L., Bryce, S. A. & Nibbs, R. J. B. Targeting cell migration in rheumatoid arthritis. Curr. Opin. Rheumatol. 27, 204–211 (2015).
de Hair, M. J. H. et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 66, 513–522 (2014).
Choi, I. Y. et al. Stromal cell markers are differentially expressed in the synovial tissue of patients with early arthritis. PLoS ONE 12, e0182751 (2017).
Filer, A. et al. Identification of a transitional fibroblast function in very early rheumatoid arthritis. Ann. Rheum. Dis. 76, 2105–2112 (2017).
Lally, F. et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 52, 3460–3649 (2005).
McGettrick, H. M. et al. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 39, 113–125 (2009).
Smith, E. et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum. 58, 1968–1973 (2008).
Allen, M. & Louise Jones, J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J. Pathol. 223, 163–177 (2011).
Klimiuk, P. A., Goronzy, J. J., Bjornsson, J., Beckenbaugh, R. D. & Weyand, C. M. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am. J. Pathol. 151, 1311–1319 (1997).
Pitzalis, C., Kelly, S. & Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 25, 334–344 (2013).
Dennis, G. et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 16, R90–R90 (2014).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Croft, A. P. et al. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis Res. Ther. 18, 270 (2016).
van Oosterhout, M. et al. Differences in synovial tissue infiltrates between anti–cyclic citrullinated peptide–positive rheumatoid arthritis and anti–cyclic citrullinated peptide–negative rheumatoid arthritis. Arthritis Rheum. 58, 53–60 (2008).
Tineke, C. et al. Alterations of the synovial T cell repertoire in anti–citrullinated protein antibody–positive rheumatoid arthritis. Arthritis Rheum. 60, 1944–1956 (2009).
Gómez-Puerta, J. A. et al. Differences in synovial fluid cytokine levels but not in synovial tissue cell infiltrate between anti-citrullinated peptide/protein antibody-positive and –negative rheumatoid arthritis patients. Arthritis Res. Ther. 15, R182–R182 (2013).
Abbot, S. E., Whish, W. J., Jennison, C., Blake, D. R. & Stevens, C. R. Tumour necrosis factor alpha stimulated rheumatoid synovial microvascular endothelial cells exhibit increased shear rate dependent leucocyte adhesion in vitro. Ann. Rheum. Dis. 58, 573–581 (1999).
Leick, M., Azcutia, V., Newton, G. & Luscinskas, F. W. Leukocyte recruitment in inflammation: basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 355, 647–656 (2014).
Tak, P. P. et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor-α monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 39, 1077–1081 (1996).
Szekanecz, Z. & Koch, A. E. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat. Rev. Rheumatol. 12, 5–13 (2016).
McNaughton, E. F. et al. Novel anti-inflammatory peptides based on chemokine–glycosaminoglycan interactions reduce leukocyte migration and disease severity in a model of rheumatoid arthritis. J. Immunol. 200, 3201–3217 (2018).
Chimen, M. et al. Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat. Med. 21, 467–475 (2015).
Kemble, S., Harford, L. & McGettrick, H. New therapeutic avenues in rheumatoid arthritis: exploring the role of the adiponectin-PEPITEM axis [abstract]. Ann. Rheum. Dis. 77 (Suppl. 1), A7 (2018).
McGettrick, H. M., Butler, L. M., Buckley, C. D., Rainger, G. E. & Nash, G. B. Tissue stroma as a regulator of leukocyte recruitment in inflammation. J. Leuk. Biol. 91, 385–400 (2012).
Parsonage, G. et al. A stromal address code defined by fibroblasts. Trends Immunol. 26, 150–156 (2005).
Luu, N.-T. et al. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cells 31, 2690–2702 (2013).
Munir, H., Luu, N. T., Clarke, L. S. C., Nash, G. B. & McGettrick, H. M. Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS ONE 11, e0155161 (2016).
McGettrick, H. M. et al. Functional pathways in endothelial cells are differentially regulated by fibroblasts from patients with RA and resolving disease [abstract]. Ann. Rheum. Dis. 74 (Suppl. 1), A57 (2015).
Davis, G. E., Norden, P. R. & Bowers, S. L. K. Molecular control of capillary morphogenesis and maturation by recognition and remodeling of the extracellular matrix: functional roles of endothelial cells and pericytes in health and disease. Connective Tissue Res. 56, 392–402 (2015).
Alon, R. & Nourshargh, S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat. Immunol. 14, 14–15 (2012).
Slowikowski, K., Wei, K., Brenner, M. B. & Raychaudhuri, S. Functional genomics of stromal cells in chronic inflammatory diseases. Curr. Opin. Rheumatol. 30, 65–71 (2018).
Williams, B., Dharmapatni, A. & Crotti, T. Intracellular apoptotic pathways: a potential target for reducing joint damage in rheumatoid arthritis. Inflamm Res. 67, 219–231 (2018).
Croft, M. & Siegel, R. M. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 13, 217–233 (2017).
Cuda, C. M., Pope, R. M. & Perlman, H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat. Rev. Rheumatol. 12, 543–558 (2016).
Liu, H. & Pope, R. M. The role of apoptosis in rheumatoid arthritis. Curr. Opin. Pharmacol. 3, 317–322 (2003).
Prendergast, C. T. et al. Visualising the interaction of CD4 T cells and DCs in the evolution of inflammatory arthritis. Ann. Rheum. Dis. 77, 579–588 (2018).
Jaigirdar, S. A. et al. Sphingosine-1-phosphate promotes the persistence of activated CD4 T cells in inflamed sites. Front. Immunol. 8, 1627 (2017).
Wang, F., Tan, W., Guo, D. & He, S. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur. J. Pharmacol. 573, 230–240 (2007).
Matsuura, M., Imayoshi, T. & Okumoto, T. Effect of FTY720, a novel immunosuppressant, on adjuvant- and collagen-induced arthritis in rats. Int. J. Immunopharmacol. 22, 323–331 (2000).
Han, Y. et al. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J. Immunol. 195, 4126–4135 (2015).
Tsunemi, S. et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 136, 197–204 (2010).
Fujii, Y. et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J. Immunol. 188, 206–215 (2012).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Chiba, K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol. Ther. 108, 308–319 (2005).
Bouta, E. M. et al. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat. Rev. Rheumatol. 14, 94–106 (2018).
Bouta, E. M. et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin. Cell Dev. Biol. 38, 90–97 (2015).
Taylor, P. C. et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor a blockade in patients with rheumatoid arthritis. Arthritis Rheum. 43, 38–47 (2000).
den Broeder, A. A. et al. Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti-tumor necrosis factor-α monoclonal antibody in patients with rheumatoid arthritis. J. Rheumatol. 30, 232–237 (2003).
Herenius, M. M. J. et al. Monocyte migration to the synovium in rheumatoid arthritis patients treated with adalimumab. Ann. Rheum. Dis. 70, 1160–1162 (2011).
Mitchell, T. S., Moots, R. J. & Wright, H. L. Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin. Exp. Immunol. 189, 250–258 (2017).
Acknowledgements
The work of the authors is supported by an Arthritis Research UK Career Development Fellowship (grant number 19899 to H.M.M.) and by an Arthritis Research UK programme grant (grant number 19791 to C.D.B.). The Arthritis Research UK Rheumatoid Arthritis Pathogenesis Centre of Excellence is partially funded by Arthritis Research UK (grant number 20298); this centre is a collaboration between the University of Glasgow, the University of Newcastle and the University of Birmingham in the UK. The MRC Arthritis Research UK Centre for Musculoskeletal Ageing Research (grant number MR/P021220/1) is a collaboration between the University of Birmingham, the University of Nottingham and Oxford University in the UK.
Referee accreditation statement
Nature Reviews Rheumatology thanks S. Jalkanen, M. Perretti and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
H.M.M. researched data for this article. Both authors provided substantial contributions to discussions of its content, wrote the article and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.D.B. declares that he has received research funding from Roche. H.M.M. declares that she has received research funding from Pfizer.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buckley, C.D., McGettrick, H.M. Leukocyte trafficking between stromal compartments: lessons from rheumatoid arthritis. Nat Rev Rheumatol 14, 476–487 (2018). https://doi.org/10.1038/s41584-018-0042-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0042-4
This article is cited by
-
New genetic and epigenetic insights into the chemokine system: the latest discoveries aiding progression toward precision medicine
Cellular & Molecular Immunology (2023)
-
IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes
Experimental & Molecular Medicine (2019)
-
Berberine mitigates IL-21/IL-21R mediated autophagic influx in fibroblast-like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis
Apoptosis (2019)
-
Pathogenic stromal cells as therapeutic targets in joint inflammation
Nature Reviews Rheumatology (2018)