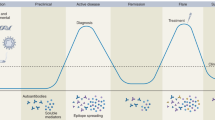
Abstract
The discovery and validation of biomarkers resulting from technological advances in the analysis of genomic, transcriptomic, lipidomic and metabolomic pathways involved in the pathogenesis of complex human diseases have led to the development of personalized and rationally designed approaches for the clinical management of such disorders. Although some of these approaches have been applied to systemic sclerosis (SSc), an unmet need remains for validated, non-invasive biomarkers to aid in the diagnosis of SSc, as well as in the assessment of disease progression and response to therapeutic interventions. Advances in global transcriptomic technology over the past 15 years have enabled the assessment of microRNAs that circulate in the blood of patients and the analysis of the macromolecular content of a diverse group of lipid bilayer membrane-enclosed extracellular vesicles, such as exosomes and other microvesicles, which are released by all cells into the extracellular space and circulation. Such advances have provided new opportunities for the discovery of biomarkers in SSc that could potentially be used to improve the design and evaluation of clinical trials and that will undoubtedly enable the development of personalized and individualized medicine for patients with SSc.
Key points
-
An urgent unmet need exists for validated non-invasive biomarkers for the diagnosis, assessment of disease activity and response to therapy of patients with systemic sclerosis (SSc).
-
Biomarkers can be used as easily measurable surrogate markers for clinical end points to aid in the stratification of patients for clinical trials.
-
Current biomarkers for SSc include cutaneous induration and assessment of serum autoantibodies and nailfold capillaroscopic patterns, although only cutaneous induration has been validated for diagnosis, prognosis or response to treatment.
-
Extracellular vesicles (EVs) contain a large number of macromolecules that reflect the physiological or pathological state of the cells of origin, rendering them a novel and valuable source of biomarkers.
-
Transcriptomic and proteomic analyses of EVs isolated from patients with SSc could provide biomarkers for diagnosis, classification and assessment of disease activity, prognosis and therapeutic response.
-
Novel biomarkers will be of value in the provision of precision and personalized medical care to patients with SSc.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Snyder, M., Du, J. & Gerstein, M. Personal genome sequencing: current approaches and challenges. Genes Dev. 24, 423–431 (2010).
Snyder, M., Weissman, S. & Gerstein, M. Personal phenotypes to go with personal genomes. Mol. Syst. Biol. 5, 273 (2009).
Laufer, V. A., Chen, J. Y., Langefeld, C. D. & Bridges, S. L. Jr. Integrative approaches to understanding the pathogenic role of genetic variation in rheumatic diseases. Rheum. Dis. Clin. North Am. 43, 449–466 (2017).
Streeter, O. E. Jr, Beron, P. J. & Iyer, P. N. Precision medicine: genomic profiles to individualize therapy. Otolaryngol. Clin. North Am. 50, 765–773 (2017).
Collins, D. C., Sundar, R., Lim, J. S. & Yap, T. A. Towards precision medicine in the clinic: from biomarker discovery to novel therapeutics. Trends Pharmacol. Sci. 38, 25–40 (2017).
Hood, L. & Flores, M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized, and participatory. Nat. Biotechnol. 29, 613–624 (2012).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Primers 1, 15002 (2015).
McCray, C. J. & Mayes, M. D. Update on systemic sclerosis. Curr. Allergy Asthma Rep. 15, 25 (2015).
Denton, C. P. & Khanna, D. Systemic sclerosis. Lancet 390, 1685–1699 (2017).
Jimenez, S. A. & Derk, C. T. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann. Intern. Med. 140, 37–50 (2004).
Varga, J. & Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 (2007).
Katsumoto, T. R., Whitfield, M. L. & Connolly, M. K. The pathogenesis of systemic sclerosis. Annu. Rev. Pathol. 6, 509–537 (2011).
Ciechomska, M., van Laar, J. & O’Reilly, S. Current frontiers in systemic sclerosis pathogenesis. Exp. Dermatol. 24, 401–406 (2015).
Stern, E. P. & Denton, C. P. The pathogenesis of systemic sclerosis. Rheum. Dis. Clin. North Am. 41, 367–382 (2015).
Pattanaik, D. et al. Pathogenesis of systemic sclerosis. Front. Immunol. 6, 272 (2015).
Young, A. & Khanna, D. Systemic sclerosis: a systemic review on therapeutic management from 2011 to 2014. Curr. Opin. Rheumatol. 27, 241–248 (2015).
Nagaraja, V., Denton, C. P. & Khanna, D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology 54, 1944–1953 (2015).
Mendoza, F. A., Mansoor, M. & Jimenez, S. A. Treatment of rapidly progressive systemic sclerosis: current and future perspectives. Expert Opin. Orphan Drugs 4, 31–47 (2016).
Mayes, M. D. et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 48, 2246–2255 (2003).
Steen, V. D. & Medsger, T. A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 66, 940–944 (2007).
Barnes, J. & Mayes, M. D. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr. Opin. Rheumatol. 24, 165–170 (2012).
Hummers, L. K. The current state of biomarkers in systemic sclerosis. Curr. Rheumatol. Rep. 12, 34–39 (2010).
Castro, S. V. & Jimenez, S. A. Biomarkers in systemic sclerosis. Biomark. Med. 4, 133–147 (2010).
Abignano, G., Buch, M., Emery, P. & Del Galdo, F. Biomarkers in the management of scleroderma: an update. Curr. Rheumatol. Rep. 13, 4–12 (2011).
Castelino, F. V. & Varga, J. Current status of systemic sclerosis biomarkers: applications for diagnosis, management and drug development. Expert Rev. Clin. Immunol. 9, 1077–1090 (2013).
Affandi, A. J., Radstake, T. R. & Marut, W. Update on biomarkers in systemic sclerosis: tools for diagnosis and treatment. Semin. Immunopathol. 37, 475–487 (2015).
Hasegawa, M. Biomarkers in systemic sclerosis: their potential to predict clinical courses. J. Dermatol. 43, 29–38 (2016).
Ligon, C. & Hummers, L. K. Biomarkers in scleroderma: progressing from association to clinical utility. Curr. Rheumatol. Rep. 18, 17 (2016).
Manetti, M. Emerging biomarkers in systemic sclerosis. Curr. Opin. Rheumatol. 28, 606–612 (2016).
NIH Definitions Working Group in Biomarkers and Surrogate Endpoints: Clinical Research and Applications: Proceedings of the NIH-FDA Conference, Bethesda, MD, 15–16 April 1999, in ICS 1205, 1e (International Congress) Ch. 1 (ed. Downing, G.) 1–9 (Elsevier, Amsterdam, 2000).
Lesko, L. J. & Atkinson, A. J. Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu. Rev. Toxicol. 41, 347–366 (2001).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Anderson, J. E. et al. Methods and biomarkers for the diagnosis and prognosis of cancer and other diseases: towards personalized medicine. Drug. Resist. Updat. 9, 198–210 (2006).
Collins, C. D. et al. The application of genomic and proteomic technologies in predictive, preventive and personalized medicine. Vascul. Pharmacol. 45, 258–267 (2006).
Isserlin, R. & Emili, A. Nine steps to proteomic wisdom: a practical guide to using protein-protein interaction networks and molecular pathways as a framework for interpreting disease proteomic profiles. Proteom. Clin. Appl. 1, 1156–1168 (2007).
Kostka, D. & Spang, R. Finding disease specific alterations in the co-expression of genes. Bioinformatics 20 (Suppl. 1), S32–S36 (2004).
Xu, M. et al. An integrative approach to characterize disease-specific pathways and their coordination: a case study in cancer. BMC Genomics 9 (Suppl. 1), S12 (2008).
Ross, J. S. Biomarkers and drug development 2009. Expert Opin. Med. Diagn. 3, 471–478 (2009).
Wagner, J. A. Overview of biomarkers and surrogate endpoints in drug development. Dis. Markers 18, 41–46 (2002).
Colburn, W. A. & Lee, J. W. Biomarkers, validation and pharmacokinetic-pharmacodynamic modelling. Clin. Pharmacokinet. 42, 997–1022 (2003).
Venitz, J. Using exposure-response and biomarkers to streamline drug development. Ernst Schering Res. Found. Workshop 59, 47–63 (2007).
Sarker, D. & Workman, P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv. Cancer Res. 96, 213–268 (2007).
Hollebecque, A., Massard, C. & Soria, J. C. Implementing precision medicine initiatives in the clinic: a new paradigm in drug development. Curr. Opin. Oncol. 26, 340–306 (2014).
Carrigan, P. & Krahn, T. Impact of biomarkers on personalized medicine. Handb. Exp. Pharmacol. 232, 285–311 (2016).
Fleming, T. R., DeGruttola, V., & DeMets, D. L. Surrogate endpoints. AIDS Clin. Rev. 1997–1998, 129–143 (1998).
Lafyatis, R. & Jimenez, S. A. in Scleroderma: From Pathogenesis to Comprehensive Management Ch. 16 (eds Varga, J. et al.) 245–260 (Springer, New York, 2017).
Hindorff, L. A. et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl Acad. Sci. USA 106, 9362–9367 (2009).
Wray, N. R. et al. Pitfalls of predicting complex traits from SNPs. Nat. Rev. Genet. 14, 507–515 (2013).
Peters, B. A. et al. Accurate whole-genome sequencing and haplotyping from 20 human cells. Nature 487, 190–195 (2012).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Kurkurba, K. R. & Montgomery, S. B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 951–969 (2015).
Favicchio, R. et al. Strategies in functional proteomics: Unveiling the pathways to precision oncology. Cancer Lett. 382, 86–94 (2016).
Zhou, L. et al. Clinical proteomics-driven precision medicine for targeted therapy: current overview and future perspectives. Expert Rev. Proteom. 13, 367–381 (2016).
Honda, K. et al. Proteomic approaches to the discovery of cancer biomarkers for early detection and personalized medicine. Jpn J. Clin. Oncol. 43, 103–109 (2013).
Huang, L., Michael, S. A., Chen, Y. & Wu, H. Current advances in highly multiplexed antibody-based single-cell proteomic measurements. Chem. Asian J. 12, 1680–1691 (2017).
Hathout, Y. Proteomic methods for biomarker discovery and validation. Are we there yet? Expert Rev. Proteom. 12, 329–331 (2015).
Richens, J. L., Lunt, E. A., Sanger, D., McKenzie, G. & O’Shea, P. Avoiding nonspecific interactions in studies of the plasma proteome: practical solutions to prevention of nonspecific interactions for label-free detection of low-abundance plasma proteins. J. Proteome Res. 8, 5103–5110 (2009).
Bruderer, R. et al. New targeted approaches for the quantification of data-independent acquisition mass spectrometry. Proteomics 17, 1700021 (2017).
Anderson, N. L. & Anderson, N. G. The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell. Proteom. 1, 845–867 (2002).
Garabedian, A. et al. Towards discovery and targeted peptide biomarker detection using nanoESI-TIMS-TOF MS. J. Am. Soc. Mass Spectrom. https://doi.org/10.1007/s13361-017-1787-8 (2017).
Ishizaki, J. et al. Targeted proteomics reveals promising biomarkers of disease activity and organ involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res. Ther. 19, 218 (2017).
Nie, S. et al. Deep-dive targeted quantification for ultrasensitive analysis of proteins in nondepleted human blood plasma/serum and tissues. Anal. Chem. 89, 9139–9146 (2017).
Kuster, B., Schirle, M., Mallick, P. & Aebersold, R. Scoring proteomes with proteotypic peptide probes. Nat. Rev. Mol. Cell Biol. 6, 577–583 (2005).
Mallick, P. et al. Computational prediction of proteotypic peptides for quantitative proteomics. Nat. Biotechnol. 25, 125–131 (2007).
Ellington, A. D. & Szostak, J. W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA Ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Gramolini, A., Lau, E. & Lui, P. P. Identifying low-abundance biomarkers: aptamer-based proteomics potentially enables more sensitive detection in cardiovascular diseases. Circulation 134, 286–289 (2016).
Yoshida, Y., Waga, I. & Horii, K. Quantitative and sensitive protein detection strategies based on aptamers. Proteom. Clin. Appl. 6, 574–580 (2012).
Thiviyanathan, V. & Gorenstein, D. G. Aptamers and the next generation of diagnostic reagents. Proteom. Clin. Appl. 6, 563–573 (2012).
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282, 790–795 (1999).
Perez-Gracia, J. L. Strategies to design clinical studies to identify predictive biomarkers in cancer research. Cancer Treat. Rev. 53, 79–97 (2017).
Wilhelm-Benartzi, C. S. et al. Challenges and methodology in the incorporation of biomarkers in cancer clinical trials. Crit. Rev. Oncol. Hematol. 110, 49–61 (2017).
Chau, C. H., Rixe, O., McLeod, H. & Figg, W. D. Validation of analytic methods for biomarkers used in drug development. Clin. Cancer Res. 14, 5967–5976 (2008).
U.S. Food & Drug Administration. Guidance for industry — pharmacogenomics data submissions. U.S. Food & Drug Administration https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079849.pdf (2005).
Goodsaid, F. & Frueh, F. Biomarker qualification pilot process at the U.S. Food and Drug Administration. AAPS J. 9, E105–108 (2007).
Lesko, L. J. & Atkinson Jr, A. J. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Pharmacol. Toxicol. 41, 347–366 (2001).
Boers, M., Brooks, P., Strand, C. V. & Tugwell, P. The OMERACT filter for outcome measures in rheumatology. J. Rheumatol. 25, 198–199 (2004).
Lassere, M. A users guide to measurement in medicine. Osteoarthritis Cartilage 14 (Suppl. 1), 10–14 (2006).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
U.S. Food & Drug Administration. Guidance for industry and FDA staff — qualification process for drug development tools. U.S. Food & Drug Administration http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm230597.pdf (2014).
Seibold, J. R. & McCloskey, D. A. Skin involvement as a relevant outcome measure in clinical trials of systemic sclerosis. Curr. Opin. Rheumatol. 9, 571–575 (1997).
Merkel, P. A. et al. OMERACT 6. Current status of outcome measure development for clinical trials in systemic sclerosis: report from OMERACT 6. J. Rheumatol. 30, 1630–1647 (2003).
Furst, D. et al. Systemic sclerosis — continuing progress in developing clinical measures of response. J. Rheumatol. 34, 1194–1200 (2007).
Khanna, D. & Merkel, P. A. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr. Rheumatol. Rep. 9, 151–157 (2007).
Khanna, D. et al. The American College of Rheumatology provisional composite response index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 68, 299–311 (2016).
Kahaleh, M. B. et al. A modified scleroderma skin scoring method. Clin. Exp. Rheumatol. 4, 367–369 (1986).
Furst, D. E. et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J. Rheumatol. 25, 84–88 (1998).
Steen, V. D. & Medsger, T. A. Jr. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 44, 2828–2835 (2001).
Kaldas, M. et al. Sensitivity to change of the modified Rodnan skin score in diffuse systemic sclerosis — assessment of individual body sites in two large randomized controlled trials. Rheumatology 48, 1143–1146 (2009).
Ziemek, J. et al. The relationship between skin symptoms and the scleroderma modification of the health assessment questionnaire, the modified Rodnan skin score, and skin pathology in patients with systemic sclerosis. Rheumatology 55, 911–917 (2016).
Khanna, D. et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2, 11–18 (2017).
Kissin, E. Y. et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 55, 603–609 (2006).
Merkel, P. A. et al. Validity, reliability, and feasibility of durometers measurements of scleroderma skin disease in a multicenter treatment trail. Arthritis Rheum. 59, 699–705 (2008).
Moore, T. L., Lunt, M., McManus, B., Anderson, M. E. & Herrick, A. L. Seventeen-point dermal ultrasound scoring system — a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology 42, 1559–1563 (2003).
Abignano, G. & Del Galdo, F. Quantitating skin fibrosis: innovative strategies and their clinical implications. Curr. Rheumatol. Rep. 16, 404 (2014).
Santiago, T. et al. A preliminary study using virtual touch imaging and quantification of the assessment of skin stiffness in systemic sclerosis. Clin. Exp. Rheumatol. 34 (Suppl. 100), S137–S141 (2016).
Merkel, P. A. et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: an individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 64, 3420–3429 (2012).
Moore, O. A. et al. Quantifying change in pulmonary function as a prognostic marker in systemic sclerosis-related interstitial lung disease. Clin. Exp. Rheumatol. 33 (Suppl. 91), S111–S116 (2015).
Goh, N. S. et al. Short term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 69, 1670–1678 (2017).
Campbell, R. M. & LeRoy, E. C. Pathogenesis of systemic sclerosis: a vascular hypotheses. Semin. Arthritis Rheum. 4, 351–368 (1975).
LeRoy, E. C. Systemic sclerosis. A vascular perspective. Rheum. Dis. Clin. North Am. 22, 675–694 (1996).
Fleming, J. N. & Schwartz, S. M. The pathology of scleroderma vascular disease. Rheum. Dis. Clin. North Am. 34, 41–55 (2008).
Kahaleh, B. Vascular disease in scleroderma: mechanisms of vascular injury. Rheum. Dis. Clin. North Am. 34, 57–71 (2008).
Trojanowska, M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat. Rev. Rheumatol. 6, 453–460 (2010).
Matucci-Cerinic, M., Kahaleh, B. & Wigley, F. M. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 65, 1953–1962 (2013).
Altorok, N., Wang, Y. & Kahaleh, B. Endothelial dysfunction in systemic sclerosis. Curr. Opin. Rheumatol. 26, 615–620 (2014).
Pattanaik, D., Brown, M. & Postlethwaite, A. E. Vascular involvement in systemic sclerosis (scleroderma). J. Inflamm. Res. 4, 105–125 (2011).
Herrick, A. L. Pathogenesis of Raynaud’s phenomenon. Rheumatology 44, 587–596 (2005).
Grassi, W. & De Angelis, R. Capillaroscopy: questions and answers. Clin. Rheumatol. 26, 2009–2016 (2007).
Maricq, H. R. & LeRoy, E. C. Patterns of finger capillary abnormalities in connective tissue disease by “wide-field” microscopy. Arthritis Rheum. 16, 619–628 (1973).
Herrick, A. L. & Cutolo, M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 62, 2595–2604 (2010).
Maricq, H. R. et al. Diagnostic potential of in vivo microscopy in scleroderma and related disorders. Arthritis Rheum. 23, 183–189 (1980).
Maricq, H. R., Weinberger, A. B. & LeRoy, E. C. Early detection of scleroderma-spectrum disorders by in vivo capillary microscopy: a prospective study of patients with Raynaud’s phenomenon. J. Rheumatol. 9, 289–291 (1983).
Cutolo, M. et al. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat. Rev. Rheumatol. 6, 578–587 (2010).
Chen, Z. Y. et al. Association between fluorescent antinuclear antibodies, capillary patterns, and clinical features in scleroderma spectrum disorders. Am. J. Med. 77, 812–822 (1984).
Caramaschi, P. et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumtology 46, 1566–1569 (2007).
Cutolo, M. et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology 43, 719–726 (2004).
Smith, V. et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann. Rheum. Dis. 71, 1636–1639 (2012).
Sulli, A. et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum. 64, 821–825 (2012).
Bredemeier, M. et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J. Rheumatol. 31, 286–294 (2004).
Hofstee, H. M. et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann. Rheum. Dis. 68, 191–195 (2009).
Sebastiani, M. et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicenter validation study. Ann. Rheum. Dis. 71, 67–70 (2012).
Lambova, S. & Muller-Ladner, U. Capillaroscopic findings in systemic sclerosis — are they associated with disease duration and presence of digital ulcers. Discov. Med. 12, 413–418 (2011).
Cutolo, M., Pizzorni, C., Sulli, A. & Smith, V. Early diagnostic and predictive value of capillaroscopy in systemic sclerosis. Curr. Rheumatol. Rev. 9, 249–253 (2013).
Cutolo, M. et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: A multicenter prospective cohort study. Arthritis Rheumatol. 68, 2527–2539 (2016).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 65, 2737–2747 (2013).
Nihtyanova, S. I. & Denton, C. P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 6, 112–116 (2010).
Villalta, D. et al. Diagnostic accuracy and predictive value of extended autoantibody profile in systemic sclerosis. Autoimmun. Rev. 12, 114–120 (2012).
Domsic, R. T. Scleroderma: the role of serum autoantibodies in defining specific clinical phenotypes and organ system involvement. Curr. Opin. Rheumatol. 26, 646–652 (2014).
Sirotti, S. et al. Personalized medicine in rheumatology: the paradigm of serum autoantibodies. Auto. Immun. Highlights 8, 10 (2017).
Mueller, M. et al. Relation of nailfold capillaries and autoantibodies to mortality in patients with Raynaud phenomenon. Circulation 133, 509–517 (2016).
Sulli, A. et al. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J. Rheumatol. 40, 634–639 (2013).
Xu, G. J. et al. Systemic autoantigen analysis identifies a distinct subset of scleroderma with coincident cancer. Proc. Natl Acad. Sci. USA 113, 57526–57534 (2016).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 3, e2696 (2008).
Sargent, J. L. & Whitfield, M. L. Capturing the heterogeneity in systemic sclerosis with genome-wide expression profiling. Expert Rev. Clin. Immunol. 7, 463–473 (2011).
Sargent, J. L. et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J. Invest. Dermatol. 130, 694–705 (2010).
Pendergrass, S. A. et al. Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLoS ONE 5, e12106 (2010).
Lenna, S. et al. Increased expression of endoplasmic reticulum stress and unfolded protein response genes in peripheral blood mononuclear cells from patients with limited cutaneous systemic sclerosis and pulmonary arterial hypertension. Arthritis Rheum. 65, 1357–1366 (2013).
Derrett-Smith, E. C. et al. Limited cutaneous systemic sclerosis skin demonstrates distinct molecular subsets separated by a cardiovascular development gene expression signature. Arthritis Res. Ther. 19, 156 (2017).
Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput. Biol. 11, e1004005 (2015).
Farina, G., Lafyatis, D., Lemaire, R. & Lafyatis, R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 62, 580–588 (2010).
Rice, L. M. et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 67, 3004–3015 (2015).
Lofgren, S. et al. Integrated multicohort analysis of systemic sclerosis identifies robust transcriptional signature of disease severity. JCI Insight 1, e89073 (2016).
Taroni, J. N. et al. Molecular characterization of systemic sclerosis esophageal pathology identifies inflammatory and proliferative signatures. Arthritis Res. Ther. 17, 194 (2015).
Taroni, J. N. et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 9, 27 (2017).
Hinchcliff, M. et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J. Invest. Dermatol. 133, 1979–1989 (2013).
Chakravarty, E. F. et al. Gene expression changes reflect clinical response in placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Arthritis Res. Ther. 17, 159 (2015).
Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125, 2795–2807 (2015).
Taroni, J. N., Martyanov, V., Mahoney, J. M. & Whitfield, M. L. A functional genomic meta-analysis of clinical trials in systemic sclerosis: toward precision medicine and combination therapy. J. Clin. Invest. Dermatol. 137, 1033–1041 (2017).
Etheridge, A. et al. The complexity, function and application of RNA in circulation. Front. Genet. 4, 115 (2013).
Etheridge, A. et al. Extracellular microRNA: a new source of biomarkers. Mutat. Res. 717, 85–90 (2011).
Witwer, K. W. Circulating microRNA biomarker studies: pitfalls and potential solution. Clin. Chem. 61, 56–63 (2015).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Eulalio, A., Huntzinger, E. & Izaurralde, E. Getting to the root of miRNA-mediated gene silencing. Cell 132, 9–14 (2008).
Treiber, T., Treiner, N. & Meister, G. Regulation of microRNA biogenesis and function. Thromb. Haemost. 107, 605–610 (2012).
Olive, V., Minella, A. C. & He, L. Outside the coding genome, mammalian microRNAs confer structural and functional complexity. Sci. Signal. 8, re2 (2015).
Tanaka, S. et al. Alteration of circulating miRNAs in SSc: miR-30b regulates the expression of PDGF receptor β. Rheumatology 52, 1963–1972 (2013).
Makino, K. et al. Circulating miR-142-3p levels in patients with systemic sclerosis. Clin. Exp. Dermatol. 37, 34–39 (2012).
Honda, N. et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am. J. Pathol. 182, 206–216 (2013).
Honda, N. et al. TGF-β-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblast. J. Immunol. 18, 3323–3331 (2012).
Makino, K. et al. The downregulation of microRNA let-7a contributes to the excessive expression of type I collagen in systemic and localized scleroderma. J. Immunol. 190, 3905–3915 (2013).
Sing, T. et al. microRNA-92a expression in the sera and dermal fibroblast increases in patients with scleroderma. Rheumatology 51, 1550–1556 (2012).
Wuttge, D. M. et al. Specific autoantibody profiles and disease subgroups correlate with circulating micro-RNA in systemic sclerosis. Rheumatology 54, 2100–2107 (2015).
Théry, C., Ostrowsky, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Gyorgy, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Théry, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820, 940–948 (2012).
Pant, S., Hilton, H. & Burczynski, M. E. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 83, 1484–1494 (2012).
Lotvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Hsu, V. W. & Prekeris, R. Transport at the recycling endosome. Curr. Opin. Cell Biol. 22, 528–534 (2010).
Hessvik, N. P. & Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75, 193–208 (2018).
Ratajczak, J. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856 (2006).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Gusachenko, O. N., Zenkova, M. A. & Vlassov, V. V. Nucleic acids in exosomes: disease markers and intercellular communication molecules. Biochemistry 78, 1 (2013).
Shurtleff, M. J. et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl Acad. Sci. USA 114, E8987–E8995 (2017).
Shelke, G. V., Jang, S. D., Yin, Y., Lasser, C. & Lotvall, J. Human mast cells release extracellular vesicle-associated DNA. Matters https://doi.org/10.19185/matters.201602000034 (2016).
Nemeth, A. et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci. Rep. 7, 8202 (2017).
Simpson, R. J., Lim, J. W., Moritz, R. L. & Mathivanan, S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteom. 6, 267–283 (2009).
Simpson, R. J., Jensen, S. S. & Lim, J. W. Proteomic profiling of exosomes: current perspectives. Proteomics 8, 4083–4099 (2008).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 30, 3.22.1–3.22.29 (2006).
Zeringer, E., Barta, T., Li, M. & Vlassov, A. V. Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 319–323 (2015).
Michalska-Jakubus, M., Kowal-Bielecka, O., Smith, V., Cutolo, M. & Krasowska, D. Plasma endothelial microparticles reflect the extent of capillaroscopic alterations and correlate with the severity of involvement in systemic sclerosis. Microvasc. Res. 110, 24–31 (2017).
Zhu, H., Luo, H. & Zuo, X. MicroRNAs: their involvement in fibrosis pathogenesis and use as diagnostic biomarkers in scleroderma. Exp. Mol. Med. 45, e41 (2013).
Steen, S. O. et al. The circulating cell-free microRNA profile in systemic sclerosis is distinct from both healthy controls and systemic lupus erythematosus. J. Rheumatol. 42, 214–221 (2015).
Wermuth, P. J., Piera-Velazquez, S. & Jimenez, S. A. Exosomes isolated from serum of systemic sclerosis patients display alterations in their content of profibrotic and antifibrotic microRNA and induce a profibrotic phenotype in cultured normal dermal fibroblast. Clin. Exp. Rheumatol. 35 (Suppl. 106), 21–30 (2017).
Simpson, R. J., Kalra, H. & Mathivanan, S. ExoCarta as a resource for exosomal research. J. Extracell. Vesicles. 1, 18374 (2012).
Keerthikumar, S. et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 248, 688–692 (2016).
Kalra, H. et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10, e1001450 (2012).
Kim, D. K., Lee, J., Simpson, R. J., Lotvall, J. & Gho, Y. S. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 40, 4–7 (2015).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 34, 474–490 (2015).
Schey, K. L., Luther, J. M. & Rose, K. L. Proteomics characterization of exosome cargo. Methods 87, 75–82 (2015).
Abramowicz, A., Widlak, P. & Pietrowska, M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol. Biosyst. 12, 1407–1419 (2016).
Wermuth, P. J., Piera-Velazquez, S. & Jimenez, S. A. Identification of novel systemic sclerosis biomarkers employing aptamer proteomic analysis. Rheumatology https://doi.org/10.1093/rheumatology/kex404 (2017).
Burmester, G. R., Bijlsma, J. W. J., Cutolo, M. & McInnes, I. B. Managing rheumatic and musculoskeletal diseases — past, present and future. Nat. Rev. Rheumatol. 13, 443–448 (2017).
Acknowledgements
The authors acknowledge the expert assistance of A. Pagano in the preparation of this manuscript. The work of the authors is supported in part by grants R21AR071644 (to S.A.J., P.J.W. and S.P.-V.) and R01AR19616 (to S.A.J.) from the NIH. The work of P.J.W. is supported by a Research Award from the Scleroderma Foundation.
Competing interests
The authors declare no competing interests.
Author information
Authors and Affiliations
Contributions
S.A.J. and P.J.W. researched data for the article and wrote the article. All authors contributed equally to discussions of the content of the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
EVpedia: http://student4.postech.ac.kr/evpedia2_xe/xe/
ExoCarta: http://www.exocarta.org/
Vesiclepedia: http://www.microvesicles.org/
Glossary
- Cutaneous induration
-
Thickening of the dermal and hypodermal layers of the skin as a result of oedema, inflammation or infiltration of immune cells.
- Selected reaction monitoring
-
An emerging targeted mass spectrometry technique for peptide biomarker discovery and validation that is able to quantify the hundreds to several thousands of peptides that are present in complex biofluids in a single experiment.
- Aptamers
-
Short single-stranded modified oligonucleotides that are able to bind to proteins, peptides and small molecules with extremely high specificity.
- Biomarker sensitivity
-
The ability of a biomarker to be measured with adequate precision and with a magnitude of change capable of detection.
- Biomarker specificity
-
The ability of a biomarker to distinguish between patients with different disease subtypes or between patients who do and do not respond to therapy.
Rights and permissions
About this article
Cite this article
Wermuth, P.J., Piera-Velazquez, S., Rosenbloom, J. et al. Existing and novel biomarkers for precision medicine in systemic sclerosis. Nat Rev Rheumatol 14, 421–432 (2018). https://doi.org/10.1038/s41584-018-0021-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0021-9
This article is cited by
-
Epigenetics as a versatile regulator of fibrosis
Journal of Translational Medicine (2023)
-
Periostin overexpression in scleroderma cardiac tissue and its utility as a marker for disease complications
Arthritis Research & Therapy (2022)
-
Extracellular Vesicles and Bone-Associated Cancer
Current Osteoporosis Reports (2021)
-
Pulmonary involvement in systemic sclerosis: exploring cellular, genetic and epigenetic mechanisms
Rheumatology International (2020)
-
Emerging targets of disease-modifying therapy for systemic sclerosis
Nature Reviews Rheumatology (2019)