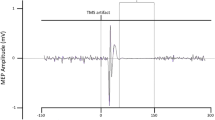
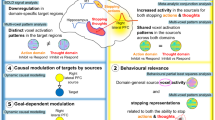
Abstract
Two decades of cross-species neuroscience research on rapid action-stopping in the laboratory has provided motivation for an underlying prefrontal–basal ganglia circuit. Here we provide an update of key studies from the past few years. We conclude that this basic neural circuit is on increasingly firm ground, and we move on to consider whether the action-stopping function implemented by this circuit applies beyond the simple laboratory stop signal task. We advance through a series of studies of increasing ‘real-worldness’, starting with laboratory tests of stopping of speech, gait and bodily functions, and then going beyond the laboratory to consider neural recordings and stimulation during moments of control presumably required in everyday activities such as walking and driving. We end by asking whether stopping research has clinical relevance, focusing on movement disorders such as stuttering, tics and freezing of gait. Overall, we conclude there are hints that the prefrontal–basal ganglia action-stopping circuit that is engaged by the basic stop signal task is recruited in myriad scenarios; however, truly proving this for real-world scenarios requires a new generation of studies that will need to overcome substantial technical and inferential challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168 (2013).
Miyake, A. et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit. Psychol. https://doi.org/10.1006/cogp.1999.0734 (2000).
Guo, Y., Schmitz, T. W., Mur, M., Ferreira, C. S. & Anderson, M. C. A supramodal role of the basal ganglia in memory and motor inhibition: meta-analytic evidence. Neuropsychologia 108, 117–134 (2018).
Verbruggen, F. et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 8, e46323 (2019).
Logan, G. D. & Cowan, W. B. On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 91, 295–327 (1984).
Sebastian, A. et al. Disentangling common and specific neural subprocesses of response inhibition. NeuroImage https://doi.org/10.1016/j.neuroimage.2012.09.020 (2013).
Sebastian, A. et al. Differential effects of age on subcomponents of response inhibition. Neurobiol. Aging https://doi.org/10.1016/j.neurobiolaging.2013.03.013 (2013).
Raud, L., Westerhausen, R., Dooley, N. & Huster, R. J. Differences in unity: the go/no-go and stop signal tasks rely on different mechanisms. NeuroImage 210, 116582 (2020).
Wessel, J. R. & Aron, A. R. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron 93, 259–280 (2017).
Aron, A. R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry 69, e55–e68 (2011). An earlier influential review of the literature on different modes of action control and their putative neural bases.
Chambers, C. D., Garavan, H. & Bellgrove, M. A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 33, 631–646 (2009).
Bari, A. & Robbins, T. W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79 (2013).
Bissett, P. G., Jones, H. M., Poldrack, R. A. & Logan, G. D. Severe violations of independence in response inhibition tasks. Sci. Adv. 7, eabf4355 (2021).
Matzke, D., Love, J. & Heathcote, A. A Bayesian approach for estimating the probability of trigger failures in the stop-signal paradigm. Behav. Res. Methods 49, 267–281 (2017).
Jana, S., Hannah, R., Muralidharan, V. & Aron, A. R. Temporal cascade of frontal, motor and muscle processes underlying human action-stopping. eLife 9, e50371 (2020). A multimodal neurophysiological study providing motivation for the temporal ‘flow’ model of processing throughout the stopping network in humans.
Smittenaar, P., Guitart-Masip, M., Lutti, A. & Dolan, R. J. Preparing for selective inhibition within frontostriatal loops. J. Neurosci. 33, 18087–18097 (2013).
Wildenberg, W. P. M., van den, Burle, B., Vidal, F., Molen, M. W. van der & Ridderinkhof, K. R. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J. Cogn. Neurosci. 22, 225–239 (2009).
Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J. & Poldrack, R. A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 27, 3743–3752 (2007).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cognit. Sci. 18, 177–185 (2014).
Aron, A. R. & Poldrack, R. A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433 (2006).
Konishi, S., Nakajima, K., Uchida, I., Sekihara, K. & Miyashita, Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur. J. Neurosci. https://doi.org/10.1046/j.1460-9568.1998.00167.x (1998).
Konishi, S. et al. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain https://doi.org/10.1093/brain/122.5.981 (1999).
Garavan, H., Ross, T. J. & Stein, E. A. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc. Natl Acad. Sci. USA 96, 8301–8306 (1999).
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J. & Robbins, T. W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 115–116 (2003). A classic study of patients with brain lesions showing that the rIFC is crucial for action-stopping.
Chambers, C. D. et al. Executive “brake failure” following deactivation of human frontal lobe. J. Cognit. Neurosci. 18, 444–455 (2006).
Verbruggen, F., Aron, A. R., Stevens, M. A. & Chambers, C. D. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl Acad. Sci. USA 107, 13966–13971 (2010).
Swann, N. C. et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage 59, 2860–2870 (2012).
Madsen, K. S. et al. Response inhibition is associated with white matter microstructure in children. Neuropsychologia 48, 854–862 (2010).
Bartoli, E., Aron, A. R. & Tandon, N. Topography and timing of activity in right inferior frontal cortex and anterior insula for stopping movement. Hum. Brain Mapp. 39, 189–203 (2018).
Suda, A. et al. Functional organization for response inhibition in the right inferior frontal cortex of individual human brains. Cereb. Cortex 30, 6325–6335 (2020).
Hannah, R., Muralidharan, V., Sundby, K. K. & Aron, A. R. Temporally-precise disruption of prefrontal cortex informed by the timing of beta bursts impairs human action-stopping. NeuroImage https://doi.org/10.1016/j.neuroimage.2020.117222 (2020).
Sundby, K. K., Jana, S. & Aron, A. R. Double blind disruption of right inferior frontal cortex with TMS reduces right frontal beta power for action-stopping. J. Neurophysiol. https://doi.org/10.1152/jn.00459.2020 (2020).
Swann, N. et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 29, 12675–12685 (2009).
Schaum, M. et al. Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans. eLife 10, 1–28 (2021).
Swick, D. & Chatham, C. H. Ten years of inhibition revisited. Front. Hum. Neurosci. 8, 115–116 (2014).
Chen, W. et al. Prefrontal-subthalamic hyperdirect pathway modulates movement inhibition in humans. Neuron 106, 579–588.e3 (2020). A study in individuals with Parkinson disease supporting the existence of a functional connection between the rIFC and the STN that is relevant to stopping.
Aron, A. R., Herz, D. M., Brown, P., Forstmann, B. U. & Zaghloul, K. Frontosubthalamic circuits for control of action and cognition. J. Neurosci. 36, 11489–11495 (2016).
Rae, C. L., Hughes, L. E., Weaver, C., Anderson, M. C. & Rowe, J. B. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. NeuroImage 86, 381–391 (2014).
Watanabe, T. et al. Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. J. Neurosci. 35, 4813–4823 (2015).
Floden, D. & Stuss, D. T. Inhibitory control is slowed in patients with right superior medial frontal damage. J. Cognit. Neurosci. 18, 1843–1849 (2006).
Roberts, R. E. & Husain, M. A dissociation between stopping and switching actions following a lesion of the pre-supplementary motor area. Cortex 63, 184–195 (2015).
Kohl, S. et al. Cortical paired associative stimulation influences response inhibition: cortico-cortical and cortico-subcortical networks. Biol. Psychiatry 85, 355–363 (2019).
Cai, W., George, J. S., Verbruggen, F., Chambers, C. D. & Aron, A. R. The role of the right presupplementary motor area in stopping action: two studies with event-related transcranial magnetic stimulation. J. Neurophysiol. 108, 380–389 (2012).
Nachev, P., Kennard, C. & Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 9, 856–869 (2008).
Thiebaut de Schotten, M., Dell’Acqua, F., Valabregue, R. & Catani, M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48, 82–96 (2012).
Rae, C. L., Hughes, L. E., Anderson, M. C. & Rowe, J. B. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci. 35, 786–794 (2015).
Inase, M., Tokuno, H., Nambu, A., Akazawa, T. & Takada, M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 833, 191–201 (1999).
Isoda, M. & Hikosaka, O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J. Neurosci. 28, 7209–7218 (2008).
Allen, C., Singh, K. D., Verbruggen, F. & Chambers, C. D. Evidence for parallel activation of the pre-supplementary motor area and inferior frontal cortex during response inhibition: a combined MEG and TMS study. R. Soc. Open Sci. 5, 171369 (2018).
Wessel, J. R. β-Bursts reveal the trial-to-trial dynamics of movement initiation and cancellation. J. Neurosci. 40, 411–423 (2020).
Errington, S. P., Woodman, G. F. & Schall, J. D. Dissociation of medial frontal β-bursts and executive control. J. Neurosci. 40, 9272–9282 (2020).
Li, B., Nguyen, T. P., Ma, C. & Dan, Y. Inhibition of impulsive action by projection-defined prefrontal pyramidal neurons. Proc. Natl Acad. Sci. USA 117, 202000523 (2020). A rodent optogenetic study confirming the importance of a dmPFC–STN pathway for action control.
Fife, K. H. et al. Causal role for the subthalamic nucleus in interrupting behavior. eLife 6, e27689 (2017).
Cavanagh, J. F. et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 14, 1462–1467 (2011).
Herz, D. M. et al. Mechanisms underlying decision-making as revealed by deep-brain stimulation in patients with Parkinson’s disease. Curr. Biol. 28, 1169–1178.e6 (2018).
Wiecki, T. V. & Frank, M. J. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol. Rev. 120, 329–355 (2013).
de Hollander, G., Keuken, M. C., van der Zwaag, W., Forstmann, B. U. & Trampel, R. Comparing functional MRI protocols for small, iron-rich basal ganglia nuclei such as the subthalamic nucleus at 7 T and 3 T. Hum. Brain Mapp. 38, 3226–3248 (2017).
Swann, N. et al. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J. Neurosci. 31, 5721–5729 (2011).
Ray, N. J. et al. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia 47, 2828–2834 (2009).
Mirabella, G. et al. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients. Cereb. Cortex 22, 1124–1132 (2012).
van den Wildenberg, W. P. M. et al. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J. Cognit. Neurosci. 18, 626–636 (2006).
Bastin, J. et al. Inhibitory control and error monitoring by human subthalamic neurons. Transl. Psychiatry 4, e439–e439 (2014).
Benis, D. et al. Response inhibition rapidly increases single-neuron responses in the subthalamic nucleus of patients with Parkinson’s disease. Cortex 84, 111–123 (2016).
Wessel, J. R. et al. Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s disease. Mov. Disord. 31, 1846–1853 (2016). A study of individuals with Parkinson disease supporting the idea that the global motor system suppression is related to basal ganglia (STN) output.
Benis, D. et al. Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. NeuroImage 91, 273–281 (2014).
Badry, R. et al. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin. Neurophysiol. 120, 1717–1723 (2009).
Hazrati, L. N. & Parent, A. Convergence of subthalamic and striatal efferents at pallidal level in primates: an anterograde double-labeling study with biocytin and PHA-L. Brain Res. 569, 336–340 (1992).
Miyachi, S. et al. Somatotopically arranged inputs from putamen and subthalamic nucleus to primary motor cortex. Neurosci. Res. 56, 300–308 (2006).
Kelly, R. M. & Strick, P. L. Macro-architecture of basal ganglia loops with the cerebral cortex: Use of rabies virus to reveal multisynaptic circuits. Prog. Brain Res. 143, 447–459 (2004).
Pasquereau, B. & Turner, R. S. A selective role for ventromedial subthalamic nucleus in inhibitory control. eLife 6, e31627 (2017). A non-human primate study supporting the idea that the ventral portion of the STN is specifically involved in action-stopping, consistent with this region receiving inputs from the lateral PFC.
Ye, Z. et al. Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson’s disease with clinical and neuroimaging measures. Hum. Brain Mapp. 37, 1026–1037 (2016).
Zandbelt, B. B., Bloemendaal, M., Neggers, S. F. W., Kahn, R. S. & Vink, M. Expectations and violations: delineating the neural network of proactive inhibitory control. Hum. Brain Mapp. 34, 2015–2024 (2013).
Zandbelt, B. B., Bloemendaal, M., Hoogendam, J. M., Kahn, R. S. & Vink, M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci. 25, 157–174 (2013).
Majid, D. S. A., Cai, W., Corey-Bloom, J. & Aron, A. R. Proactive selective response suppression is implemented via the basal ganglia. J. Neurosci. 33, 13259–13269 (2013).
Rao, J. A. et al. Disruption of response inhibition circuits in prodromal Huntington disease. Cortex 58, 72–85 (2014).
Jahfari, S., Stinear, C. M., Claffey, M., Verbruggen, F. & Aron, A. R. Responding with restraint: what are the neurocognitive mechanisms? J. Cognit. Neurosci. 22, 1479–1492 (2010).
Terra, H. et al. Prefrontal cortical projection neurons targeting dorsomedial striatum control behavioral inhibition. Curr. Biol. 30, 4188–4200.e5 (2020).
Gu, B.-M., Schmidt, R. & Berke, J. D. Globus pallidus dynamics reveal covert strategies for behavioral inhibition. eLife 9, 1–19 (2020).
Klaus, A. et al. The spatiotemporal organization of the striatum encodes action space. Neuron 95, 1171–1180.e7 (2017).
Jin, X., Tecuapetla, F. & Costa, R. M. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat. Neurosci. 17, 423–430 (2014).
Mink, J. W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996).
Coxon, J. P., Stinear, C. M. & Byblow, W. D. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 95, 3371–3383 (2006).
Cai, W., Oldenkamp, C. L. & Aron, A. R. Stopping speech suppresses the task-irrelevant hand. Brain Lang. 120, 412–415 (2012).
Pani, P. et al. Visual salience of the stop signal affects the neuronal dynamics of controlled inhibition. Sci. Rep. 8, 1–13 (2018).
Jerjian, S. J., Sahani, M. & Kraskov, A. Movement initiation and grasp representation in premotor and primary motor cortex mirror neurons. eLife 9, 1–26 (2020).
Raud, L. & Huster, R. J. The temporal dynamics of response inhibition and their modulation by cognitive control. Brain Topogr. 30, 486–501 (2017).
Schmidt, R. & Berke, J. D. A pause-then-cancel model of stopping: evidence from basal ganglia neurophysiology. Philos. Trans. R. Soc. B: Biol. Sci. https://doi.org/10.1098/rstb.2016.0202 (2017).
Schmidt, R., Leventhal, D. K., Mallet, N., Chen, F. & Berke, J. D. Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci. 16, 1118–1124 (2013).
Mallet, N. et al. Arkypallidal cells send a stop signal to striatum. Neuron 89, 308–316 (2016).
Frank, M. J., Samanta, J., Moustafa, A. A. & Sherman, S. J. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318, 1309–1312 (2007).
Heston, J. et al. Activation of subthalamic nucleus stop circuit disrupts cognitive performance. eNeuro https://doi.org/10.1523/ENEURO.0159-20.2020 (2020).
Wessel, J. R. et al. Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nat. Commun. 7, 1–10 (2016).
Ardila, A., Bernal, B. & Rosselli, M. How localized are language brain areas? A review of Brodmann areas involvement in oral language. Arch. Clin. Neuropsychol. 31, 112–122 (2016).
Stewart, L., Walsh, V., Frith, U. & Rothwell, J. C. TMS produces two dissociable types of speech disruption. NeuroImage 13, 472–478 (2001).
Xue, G., Aron, A. R. & Poldrack, R. A. Common neural substrates for inhibition of spoken and manual responses. Cereb. Cortex 18, 1923–1932 (2008).
Wagner, J., Wessel, J. R., Ghahremani, A. & Aron, A. R. Establishing a right frontal beta signature for stopping action in scalp EEG: implications for testing inhibitory control in other task contexts. J. Cognit. Neurosci. 30, 107–118 (2018).
Ghahremani, A. et al. Stopping and slowing manual and spoken responses: similar oscillatory signatures recorded from the subthalamic nucleus. Brain Lang. 176, 1–10 (2018).
Kinoshita, M. et al. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct. Funct. 220, 3399–3412 (2015).
Lüders, H. et al. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia 29, S56–S65 (1988).
Filevich, E., Kühn, S. & Haggard, P. Negative motor phenomena in cortical stimulation: Implications for inhibitory control of human action. Cortex 48, 1251–1261 (2012).
Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 10, 1–17 (2017).
Gritton, H. J. et al. Unique contributions of parvalbumin and cholinergic interneurons in organizing striatal networks during movement. Nat. Neurosci. 22, 586–597 (2019).
Rizzi, G. & Tan, K. R. Synergistic nigral output pathways shape movement. Cell Rep. 27, 2184–2198.e4 (2019).
Pamukcu, A. et al. Parvalbumin+ and Npas1+ pallidal neurons have distinct circuit topology and function. (2020) https://doi.org/10.1523/JNEUROSCI.0361-20.2020.
Ebersbach, G., Moreau, C., Gandor, F., Defebvre, L. & Devos, D. Clinical syndromes: parkinsonian gait. Mov. Disord. 28, 1552–1559 (2013).
Fischer, P. et al. Alternating modulation of subthalamic nucleus beta oscillations during stepping. J. Neurosci. 38, 5111–5121 (2018).
Bancroft, M. J. & Day, B. L. The throw-and-catch model of human gait: evidence from coupling of pre-step postural activity and step location. Front. Hum. Neurosci. 10, 635 (2016).
Potocanac, Z., Pijnappels, M., Verschueren, S., van Dieën, J. & Duysens, J. Two-stage muscle activity responses in decisions about leg movement adjustments during trip recovery. J. Neurophysiol. 115, 143–156 (2016).
Goode, C., Cole, D. M. & Bolton, D. A. E. Staying upright by shutting down? Evidence for global suppression of the motor system when recovering balance. Gait Posture 70, 260–263 (2019). An innovative study in humans attempting to study the neural basis of action-stopping in a more naturalistic gait-and-balance context.
Rydalch, G., Bell, H. B., Ruddy, K. L. & Bolton, D. A. E. Stop-signal reaction time correlates with a compensatory balance response. Gait Posture 71, 273–278 (2019).
Adam, E., Johns, T. & Sur, M. Cortico-subthalamic projections send brief stop signals to halt visually-guided locomotion. https://doi.org/10.1101/2020.02.05.936443 (2020).
van der Salm, S. M. A. et al. Distinctive tics suppression network in Gilles de la Tourette syndrome distinguished from suppression of natural urges using multimodal imaging. NeuroImage Clin. 20, 783–792 (2018).
Berman, B. D., Horovitz, S. G., Morel, B. & Hallett, M. Neural correlates of blink suppression and the buildup of a natural bodily urge. NeuroImage 59, 1441–1450 (2012).
Mazzone, S. B., Cole, L. J., Ando, A., Egan, G. F. & Farrell, M. J. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J. Neurosci. 31, 2948–2958 (2011).
Ando, A. et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 71, 323–329 (2016).
McKay, L. C., Adams, L., Frackowiak, R. S. J. & Corfield, D. R. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. NeuroImage 40, 1824–1832 (2008).
Lynn, M. T., Demanet, J., Krebs, R. M., van Dessel, P. & Brass, M. Voluntary inhibition of pain avoidance behavior: an fMRI study. Brain Struct. Funct. 221, 1309–1320 (2016).
Critchley, H. D. et al. Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates. (2015) https://doi.org/10.1371/journal.pone.0127082.
Sundby, K. K., Wagner, J. & Aron, A. R. The functional role of response suppression during an urge to relieve pain. J. Cognit. Neurosci. 31, 1404–1421 (2018).
Smith, W. K. The representation of respiratory movements in the cerebral cortex. J. Neurophysiol. 1, 55–68 (1938).
Schel, M. A. et al. Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front. Hum. Neurosci. 8, 27 (2014).
Jahanshahi, M. & Rothwell, J. C. Inhibitory dysfunction contributes to some of the motor and non-motor symptoms of movement disorders and psychiatric disorders. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160198 (2017).
de Havas, J., Gomi, H. & Haggard, P. Experimental investigations of control principles of involuntary movement: a comprehensive review of the Kohnstamm phenomenon. Exp. Brain Res. 235, 1953–1997 (2017).
Ghosh, A., Rothwell, J. & Haggard, P. Using voluntary motor commands to inhibit involuntary arm movements. Proc. Biol. Sci. 281, 20141139 (2014).
Parkinson, A., McDonagh, M. & Vidyasagar, R. Brain activation in an involuntary human action. Brain Res. 1304, 57–65 (2009).
de Havas, J., Ghosh, A., Gomi, H. & Haggard, P. Voluntary motor commands reveal awareness and control of involuntary movement. Cognition 155, 155–167 (2016).
Wessel, J. R. & Aron, A. R. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J. Neurosci. 33, 18481–18491 (2013).
Dutra, I. C., Waller, D. A. & Wessel, J. R. Perceptual surprise improves action stopping by nonselectively suppressing motor activity via a neural mechanism for motor inhibition. J. Neurosci. 38, 1482–1492 (2018).
Hampshire, A. & Sharp, D. J. Contrasting network and modular perspectives on inhibitory control. Trends Cognit. Sci. 19, 445–452 (2015).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Right inferior frontal cortex: addressing the rebuttals. Front. Hum. Neurosci. 8, 905 (2014).
Sharp, D. J. et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl Acad. Sci. USA 107, 6106–6111 (2010).
Boto, E. et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 555, 657–661 (2018).
Lacerenza, M. et al. Wearable and wireless time-domain near-infrared spectroscopy system for brain and muscle hemodynamic monitoring. Biomed. Opt. Express https://doi.org/10.1364/boe.403327 (2020).
Topalovic, U. et al. Wireless programmable recording and stimulation of deep brain activity in freely moving humans. Neuron 108, 322–334.e9 (2020). A demonstration of novel technology for wearable devices for recording and perturbing brain activity, and its integration with other behavioural recording devices.
Poldrack, R. A. Can cognitive processes be inferred from neuroimaging data? Trends Cognit. Sci. 10, 59–63 (2006).
Verbruggen, F. & Logan, G. D. Proactive adjustments of response strategies in the stop-signal paradigm. J. Exp. Psychol. 35, 835–854 (2009).
Lee, W.-T. & Kang, M.-S. Electrophysiological evidence for distinct proactive control mechanisms in a stop-signal task: an individual differences approach. Front. Psychol. 11, 1105 (2020).
Horstmann, G. The surprise-attention link: a review. Ann. N. Y. Acad. Sci. 1339, 106–115 (2015).
Liang, F. et al. Sensory cortical control of a visually induced arrest behavior via corticotectal projections. Neuron 86, 755–767 (2015).
Eisenberg, I. W. et al. Uncovering the structure of self-regulation through data-driven ontology discovery. Nat. Commun. 10, 1–13 (2019).
Neef, N. E. et al. Structural connectivity of right frontal hyperactive areas scales with stuttering severity. Brain 141, 191–204 (2018). A neuroimaging study highlighting the relationship between connectivity in right prefrontal regions of the stopping network and stuttering severity.
Eggers, K., de Nil, L. F. & van den Bergh, B. R. H. Inhibitory control in childhood stuttering. J. Fluen. Disord. 38, 1–13 (2013).
Markett, S. et al. Impaired motor inhibition in adults who stutter – evidence from speech-free stop-signal reaction time tasks. Neuropsychologia 91, 444–450 (2016).
Treleaven, S. B. & Coalson, G. A. Manual response inhibition and quality of life in adults who stutter. J. Commun. Disord. 88, 106053 (2020).
Fox, P. T. et al. A PET study of the neural systems of stuttering. Nature 382, 158–162 (1996).
Belyk, M., Kraft, S. J. & Brown, S. Stuttering as a trait or state - an ALE meta-analysis of neuroimaging studies. Eur. J. Neurosci. 41, 275–284 (2015).
Kell, C. A. et al. How the brain repairs stuttering. Brain 132, 2747–2760 (2009).
Preibisch, C. et al. Evidence for compensation for stuttering by the right frontal operculum. NeuroImage 20, 1356–1364 (2003).
Mink, J. W. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatric Neurol. 25, 190–198 (2001).
Kataoka, Y. et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 518, 277–291 (2010).
Morand-Beaulieu, S. et al. The puzzling question of inhibitory control in Tourette syndrome: a meta-analysis. Neurosci. Biobehav. Rev. 80, 240–262 (2017).
Koller, W. C. & Biary, N. M. Volitional control of involuntary movements. Mov. Disord. 4, 153–156 (1989).
Kim, S. et al. Tic suppression in children with recent-onset tics predicts 1-year tic outcome. J. Child. Neurol. 34, 757–764 (2019).
Fründt, O., Woods, D. & Ganos, C. Behavioral therapy for Tourette syndrome and chronic tic disorders. Neurol. Clin. Pract. 7, 148–156 (2017).
Ganos, C. et al. Motor cortical excitability during voluntary inhibition of involuntary tic movements. Mov. Disord. 33, 1804–1809 (2018). A study in individuals with tics demonstrating that tonic voluntary suppression of tics is associated with a global motor system suppression akin to that seen when stopping voluntary actions.
Brandt, V. C. et al. Temporal relationship between premonitory urges and tics in Gilles de la Tourette syndrome. Cortex 77, 24–37 (2016).
Ganos, C. et al. The neural correlates of tic inhibition in Gilles de la Tourette syndrome. Neuropsychologia 65, 297–301 (2014).
Peterson, B. S. et al. A functional magnetic resonance imaging study of tic suppression in tourette syndrome. Arch. Gen. Psychiatry 55, 326–333 (1998).
Zapparoli, L., Macerollo, A., Joyce, E. M., Martino, D. & Kilner, J. M. Voluntary tic suppression and the normalization of motor cortical beta power in Gilles de la Tourette syndrome: an EEG study. Eur. J. Neurosci. https://doi.org/10.1111/ejn.14548 (2019).
Ehgoetz Martens, K. A. et al. The functional network signature of heterogeneity in freezing of gait. Brain 141, 1145–1160 (2018).
Lewis, S. J. G. & Shine, J. M. The next step: a common neural mechanism for freezing of gait. Neuroscientist 22, 72–82 (2016).
Shine, J. M. et al. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin. Neurophysiol. 125, 569–576 (2014).
Pozzi, N. G. et al. Freezing of gait in Parkinson’s disease reflects a sudden derangement of locomotor network dynamics. Brain 142, 2037–2050 (2019). A study in individuals with Parkinson disease demonstrating that connectivity in a putative PFC–STN hyperdirect pathway is involved in the freezing of gait.
Matar, E. et al. Identifying the neural correlates of doorway freezing in Parkinson’s disease. Hum. Brain Mapp. 40, 2055–2064 (2019).
Chen, C. C. et al. Subthalamic nucleus oscillations correlate with vulnerability to freezing of gait in patients with Parkinson’s disease. Neurobiol. Dis. 132, 104605 (2019).
Rubinstein, T. C., Giladi, N. & Hausdorff, J. M. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov. Disord. 17, 1148–1160 (2002).
Castiglione, A. & Aron, A. R. Unwanted memory intrusions recruit broad motor suppression. J. Cogn. Neurosci. https://doi.org/10.1162/jocn_a_01642 (2020).
Castiglione, A., Wagner, J., Anderson, M. & Aron, A. R. Preventing a thought from coming to mind elicits increased right frontal beta just as stopping action does. Cereb. Cortex 29, 2160–2172 (2019). A neurophysiological study showing that stopping an unwanted thought intrusion from coming to mind is associated with a similar prefrontal beta-band signature as stopping an action.
Anderson, M. C. & Green, C. Suppressing unwanted memories by executive control. Nature 410, 366–369 (2001).
Visser, R. M. et al. Neuropsychological Mechanisms of Intrusive Thinking. In: Kalivas P. W. and Paulus M. P. (eds). Vol. 30, 124–184 (The MIT Press, 2020).
Skippen, P. et al. Reliability of triggering inhibitory process is a better predictor of impulsivity than SSRT. Acta Psychol. 192, 104–117 (2019).
Klaus, A., Alves da Silva, J. & Costa, R. M. What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci. 42, 459–483 (2019).
Nambu, A., Tokuno, H. & Takada, M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci. Res. 43, 111–117 (2002).
Osada, T. et al. An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network. J. Neurosci. 39, 2509–2521 (2019).
Hannah, R. & Jana, S. Disentangling the role of posterior parietal cortex in response inhibition. J. Neurosci. 39, 6814–6816 (2019).
Silsupadol, P., Teja, K. & Lugade, V. Reliability and validity of a smartphone-based assessment of gait parameters across walking speed and smartphone locations: body, bag, belt, hand, and pocket. Gait Posture 58, 516–522 (2017).
Cai, W., Oldenkamp, C. L. & Aron, A. R. A proactive mechanism for selective suppression of response tendencies. J. Neurosci. 31, 5965–5969 (2011).
Majid, D. S. A., Cai, W., George, J. S., Verbruggen, F. & Aron, A. R. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb. Cortex 22, 363–371 (2012).
Jahfari, S. et al. How preparation changes the need for top-down control of the basal ganglia when inhibiting premature actions. J. Neurosci. 32, 10870–10878 (2012).
Aron, A. R. & Verbruggen, F. Stop the presses: dissociating a selective from a global mechanism for stopping: research article. Psychol. Sci. 19, 1146–1153 (2008).
Mars, R. B., Piekema, C., Coles, M. G. H., Hulstijn, W. & Toni, I. On the programming and reprogramming of actions. Cereb. Cortex 17, 2972–2979 (2007).
Boecker, M. et al. When response inhibition is followed by response reengagement: an event-related fMRI study. Hum. Brain Mapp. 32, 94–106 (2011).
Isoda, M. & Hikosaka, O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 10, 240–248 (2007).
Neubert, F.-X., Mars, R. B., Buch, E. R., Olivier, E. & Rushworth, M. F. S. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc. Natl Acad. Sci. USA 107, 13240–13245 (2010).
Greenhouse, I. & Wessel, J. R. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology 50, 900–908 (2013).
Wessel, J. R. & Aron, A. R. It’s not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology 52, 472–480 (2015).
Huster, R. J., Messel, M. S., Thunberg, C. & Raud, L. The P300 as marker of inhibitory control – fact or fiction? Cortex 132, 334–348 (2020).
Atsma, J., Maij, F., Gu, C., Medendorp, W. P. & Corneil, B. D. Active braking of whole-arm reaching movements provides single-trial neuromuscular measures of movement cancellation. J. Neurosci. 38, 4367–4382 (2018).
Acknowledgements
The authors gratefully acknowledge funding support from the US National Institutes of Health (NS106822 and DA026452) and comments on the manuscript from J. Rothwell.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks D. Bolton, S. Konishi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Offline transcranial magnetic stimulation
-
(TMS). Non-invasive brain stimulation eliciting long-lasting after-effects. Typically delivered before a participant undergoes behavioural testing or neuroimaging.
- Electrocorticography
-
(ECoG). Invasive electrophysiological technique that uses electrodes placed directly on the exposed surface of the brain to record electrical activity from the cerebral cortex.
- Hyperdirect pathway
-
Pathway connecting cortical areas directly to the subthalamic nucleus, bypassing the striatum.
- Electromyography
-
(EMG). Electrophysiological technique that uses electrodes placed on the skin over a muscle to record its electrical activity.
- Online TMS
-
Non-invasive brain stimulation eliciting short-lived after-effects. Typically delivered while a participant undergoes behavioural testing or neuroimaging.
- Conflict resolution
-
In the motor domain, the process of resolving competition between competing action plans.
- Go–no-go task
-
A paradigm where the participant is required to perform speeded responses to a go cue and to withhold a response following a no-go cue.
- Global motor system suppression
-
Suppression of motor system excitability detected in task-irrelevant muscle representations when stopping with another effector. Relies on transcranial magnetic stimulation and electromyography methods.
- Premanifest Huntington disease
-
The presymptomatic phase of the disease in an individual carrying the genetic mutation causing it.
- Agonist muscle
-
The muscles that, when activated, are primarily responsible for causing movement about a joint.
- Gait
-
The normal pattern of limb movements underpinning locomotion.
- Kohnstamm phenomenon
-
A long-lasting (10–60-s) involuntary muscle contraction that develops after a sustained, voluntary isometric contraction: after pushing your arm against a wall for a long period, you experience your arm rising.
- Antagonist muscles
-
The muscles that, when activated, oppose the movement caused by the agonist muscles about a joint.
Rights and permissions
About this article
Cite this article
Hannah, R., Aron, A.R. Towards real-world generalizability of a circuit for action-stopping. Nat Rev Neurosci 22, 538–552 (2021). https://doi.org/10.1038/s41583-021-00485-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-021-00485-1
This article is cited by
-
Uncovering the neurophysiology of mood, motivation and behavioral symptoms in Parkinson’s disease through intracranial recordings
npj Parkinson's Disease (2023)
-
Comparing anticipatory and stop-signal response inhibition with a novel, open-source selective stopping toolbox
Experimental Brain Research (2023)
-
Ventral and dorsal aspects of the inferior frontal-occipital fasciculus support verbal semantic access and visually-guided behavioural control
Brain Structure and Function (2023)
-
A Novel Mouse Model for Polysynaptic Retrograde Tracing and Rabies Pathological Research
Cellular and Molecular Neurobiology (2023)