Abstract
A rapidly expanding and clinically distinct group of CNS diseases are caused by pathogenic autoantibodies that target neuroglial surface proteins. Despite immunotherapy, patients with these neuroglial surface autoantibody (NSAb)-mediated diseases often experience clinical relapse, high rates of long-term morbidity and adverse effects from the available medications. Fundamentally, the autoantigen-specific B cell lineage leads to production of the pathogenic autoantibodies. These autoantigen-specific B cells have been consistently identified in the circulation of patients with NSAb-mediated diseases, accompanied by high serum levels of autoantigen-specific antibodies. Early evidence suggests that these cells evade well-characterized B cell tolerance checkpoints. Nearer to the site of pathology, cerebrospinal fluid from patients with NSAb-mediated diseases contains high levels of autoantigen-specific B cells that are likely to account for the intrathecal synthesis of these autoantibodies. The characteristics of their immunoglobulin genes offer insights into the underlying immunobiology. In this Review, we summarize the emerging knowledge of B cells across the NSAb-mediated diseases. We review the evidence for the relative contributions of germinal centres and long-lived plasma cells as sources of autoantibodies, discuss data that indicate migration of B cells into the CNS and summarize insights into the underlying B cell pathogenesis that are provided by therapeutic effects.
Key points
-
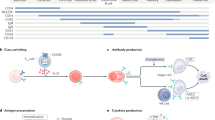
Autoantigen-specific B cells that target neuroglial surface proteins are responsible for the production of pathogenic neuroglial surface autoantibodies (NSAbs) in an increasing variety of antibody-mediated CNS diseases.
-
Pathogenic NSAbs are typically found at higher concentrations in the serum than in the cerebrospinal fluid.
-
Current evidence suggests that autoreactive B cells evade early B cell tolerance checkpoints in NSAb-mediated CNS diseases.
-
Enrichment of autoantigen-specific B cells in the cerebrospinal fluid in patients with NSAb-mediated diseases suggests that they migrate into this compartment and secrete pathogenic IgGs intrathecally.
-
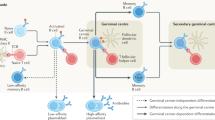
Experimental and clinical evidence implicates both ongoing germinal centre reactions and long-lived plasma cells in the propagation of autoantibody production in NSAb-mediated diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Varley, J., Vincent, A. & Irani, S. R. Clinical and experimental studies of potentially pathogenic brain-directed autoantibodies: current knowledge and future directions. J. Neurol. 262, 1081–1095 (2015).
Ramanathan, S., Al-Diwani, A., Waters, P. & Irani, S. R. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J. Neurol. https://doi.org/10.1007/s00415-019-09590-9 (2019).
Titulaer, M. J. et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165 (2013).
Thompson, J. et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141, 348–356 (2018).
Kreye, J. et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain 139, 2641–2652 (2016).
Patterson, K. R., Dalmau, J. & Lancaster, E. Mechanisms of Caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Ann. Neurol. 83, 40–51 (2018).
Petit-Pedrol, M. et al. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain 141, 3144–3159 (2018).
Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm. 6, e547 (2019).
Takata, K. et al. Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI insight 4, e127167 (2019).
Jones, B. E. et al. Autoimmune receptor encephalitis in mice induced by active immunization with conformationally stabilized holoreceptors. Sci. Transl Med. 11, eaaw0044 (2019).
Ramberger, M. et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 143, 1731–1745 (2020).
Kornau, H.-C. et al. Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann. Neurol. 87, 405–418 (2020).
Duan, T. & Verkman, A. S. Experimental animal models of aquaporin-4-IgG-seropositive neuromyelitis optica spectrum disorders: progress and shortcomings. Brain Pathol. 30, 13–25 (2020).
Dalmau, J. et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 7, 1091–1098 (2008).
Irani, S. R. et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133, 2734–2748 (2010).
van Sonderen, A. et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87, 521–528 (2016).
Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 17, 281–294 (2017).
Meffre, E. & O’Connor, K. C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019).
Schatz, D. G. & Ji, Y. Recombination centres and the orchestration of V(D)J recombination. Nat. Rev. Immunol. 11, 251–263 (2011).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Tiller, T. et al. Autoreactivity in human IgG+ memory B cells. Immunity 26, 205–213 (2007).
Tsuiji, M. et al. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 203, 393–400 (2006).
Meffre, E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann. NY Acad. Sci. 1246, 1–10 (2011).
Benschop, R. J., Brandl, E., Chan, A. C. & Cambier, J. C. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J. Immunol. 167, 4172–4179 (2001).
Wing, J. B. & Sakaguchi, S. Foxp3(+) T(reg) cells in humoral immunity. Int. Immunol. 26, 61–69 (2014).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Lesley, R. et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441–453 (2004).
Baumgarth, N. How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunol. Rev. 255, 82–94 (2013).
Chan, T. D. et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity 37, 893–904 (2012).
Burnett, D. L. et al. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360, 223–226 (2018).
Wilson, R. et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain 141, 1063–1074 (2018).
Makuch, M. et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann. Neurol. 83, 553–561 (2018).
Winklmeier, S. et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol. Neuroimmunol. Neuroinflamm. 6, 625 (2019).
Cotzomi, E. et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 142, 1598–1615 (2019).
Lee, J.-Y. et al. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann. Clin. Transl Neurol. 3, 443–454 (2016).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Samuels, J., Ng, Y.-S., Coupillaud, C., Paget, D. & Meffre, E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201, 1659–1667 (2005).
Kinnunen, T. et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Invest. 123, 2737–2741 (2013).
Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37, 1141–1149 (2000).
Leyendeckers, H. et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur. J. Immunol. 29, 1406–1417 (1999).
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6, 741–750 (2006).
Sharma, R. et al. Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Ann. Clin. Transl Neurol. 5, 935–951 (2018).
Chihara, N. et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl Acad. Sci. USA 108, 3701–3706 (2011).
Kowarik, M. C. et al. CNS aquaporin-4-specific B cells connect with multiple B-cell compartments in neuromyelitis optica spectrum disorder. Ann. Clin. Transl Neurol. 4, 369–380 (2017).
Hara, M. et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology 90, e1386–e1394 (2018).
Jarius, S., Franciotta, D., Bergamaschi, R., Wildemann, B. & Wandinger, K.-P. Immunoglobulin M antibodies to aquaporin-4 in neuromyelitis optica and related disorders. Clin. Chem. Lab. Med. 48, 659–663 (2010).
Castillo-Gomez, E. et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol. Psychiatry 22, 1776–1784 (2017).
Barth, W. F., Wochner, R. D., Waldmann, T. A. & Fahey, J. L. Metabolism of human gamma macroglobulins. J. Clin. Invest. 43, 1036–1048 (1964).
Bohannon, C. et al. Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat. Commun. 7, 11826 (2016).
Tabata, E. et al. Immunopathological significance of ovarian teratoma in patients with anti-N-methyl-d-aspartate receptor encephalitis. Eur. Neurol. 71, 42–48 (2014).
Chefdeville, A. et al. Immunopathological characterization of ovarian teratomas associated with anti-N-methyl-D-aspartate receptor encephalitis. Acta Neuropathol. Commun. 7, 38 (2019).
Nolan, A., Buza, N., Margeta, M. & Rabban, J. T. Ovarian teratomas in women with anti-N-methyl-D-aspartate receptor encephalitis: topography and composition of immune cell and neuroglial populations is compatible with an autoimmune mechanism of disease. Am. J. Surg. Pathol. 43, 949–964 (2019).
Tuzun, E. et al. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 118, 737–743 (2009).
Day, G. S., Laiq, S., Tang-Wai, D. F. & Munoz, D. G. Abnormal neurons in teratomas in NMDAR encephalitis. JAMA Neurol. 71, 717–724 (2014).
Irani, S. R. et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133, 1655–1667 (2010).
Havenar-Daughton, C. et al. CXCL13 is a plasma biomarker of germinal center activity. Proc. Natl Acad. Sci. USA 113, 2702–2707 (2016).
Leypoldt, F. et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol. 72, 180–186 (2015).
Byun, J.-I. et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. J. Neuroimmunol. 297, 141–147 (2016).
Liba, Z. et al. Anti-N-methyl-D-aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J. Neuroinflammation 13, 55 (2016).
Lin, Y.-T., Yang, X., Lv, J.-W., Liu, X.-W. & Wang, S.-J. CXCL13 is a biomarker of anti-leucine-rich glioma-inactivated protein 1 encephalitis patients. Neuropsychiatr. Dis. Treat. 15, 2909–2915 (2019).
Dale, R. C. et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 83, 142–150 (2014).
Damato, V., Evoli, A. & Iorio, R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 73, 1342–1348 (2016).
Cree, B. A. C. et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64, 1270–1272 (2005).
Kim, S.-H., Kim, W., Li, X. F., Jung, I.-J. & Kim, H. J. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch. Neurol. 68, 1412–1420 (2011).
Valentino, P., Marnetto, F., Granieri, L., Capobianco, M. & Bertolotto, A. Aquaporin-4 antibody titration in NMO patients treated with rituximab: a retrospective study. Neurol. Neuroimmunol. Neuroinflamm. 4, e317 (2017).
Pellkofer, H. L. et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 76, 1310–1315 (2011).
Brown, J. W. L. et al. Long-term remission with rituximab in refractory leucine-rich glioma inactivated 1 antibody encephalitis. J. Neuroimmunol. 271, 66–68 (2014).
Irani, S. R., Gelfand, J. M., Bettcher, B. M., Singhal, N. S. & Geschwind, M. D. Effect of rituximab in patients with leucine-rich, glioma-inactivated 1 antibody-associated encephalopathy. JAMA Neurol. 71, 896–900 (2014).
Waters, P. et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch. Neurol. 65, 913–919 (2008).
Kim, T.-J. et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann. Neurol. 81, 183–192 (2017).
van Sonderen, A. et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann. Neurol. 81, 193–198 (2017).
Binks, S. et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 141, 2263–2271 (2018).
Mueller, S. H. et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann. Neurol. 83, 863–869 (2018).
Gontika, M. & Anagnostouli, M. Human leukocyte antigens-immunogenetics of neuromyelitis optica or Devic′s disease and the impact on the immunopathogenesis, diagnosis and treatment: a critical review. Neuroimmunol. Neuroinflamm. 1, 44–50 (2014).
Gitlin, A. D., Shulman, Z. & Nussenzweig, M. C. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 509, 637–640 (2014).
Shulman, Z. et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 345, 1058–1062 (2014).
Thaler, F. S. et al. Abundant glutamic acid decarboxylase (GAD)-reactive B cells in gad-antibody-associated neurological disorders. Ann. Neurol. 85, 448–454 (2019).
Mumtaz, I. M. et al. Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: implications for the treatment of autoimmunity. J. Autoimmun. 39, 180–188 (2012).
Alexander, T. et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 113, 214–223 (2009).
Burt, R. K. et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology 93, e1732–e1741 (2019).
Jarius, S. et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain 131, 3072–3080 (2008).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Sato, D. K. et al. Cerebrospinal fluid aquaporin-4 antibody levels in neuromyelitis optica attacks. Ann. Neurol. 76, 305–309 (2014).
Icoz, S. et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int. J. Neurosci. 120, 71–75 (2010).
Araki, M. Blockade of IL-6 signaling in neuromyelitis optica. Neurochem. Int. 130, 104315 (2019).
Lee, W.-J. et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics 13, 824–832 (2016).
Scheibe, F. et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology 88, 366–370 (2017).
Shin, Y.-W., Lee, S.-T., Kim, T.-J., Jun, J.-S. & Chu, K. Bortezomib treatment for severe refractory anti-NMDA receptor encephalitis. Ann. Clin. Transl Neurol. 5, 598–605 (2018).
Keddie, S. et al. Plasma cell depletion with bortezomib in the treatment of refractory N-methyl-d-aspartate (NMDA) receptor antibody encephalitis. Rational developments in neuroimmunological treatment. Eur. J. Neurol. 25, 1384–1388 (2018).
Taylor, J. & Irani, S. R. Bortezomib for neuromyelitis optica spectrum disorder: a new therapeutic option for the more severe forms? JAMA Neurol. 75, 129 (2018).
Kishimoto, T. The biology of interleukin-6. Blood 74, 1–10 (1989).
Engelhardt, B. et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 132, 317–338 (2016).
van Sonderen, A. et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87, 1449–1456 (2016).
Majed, M., Fryer, J. P., McKeon, A., Lennon, V. A. & Pittock, S. J. Clinical utility of testing AQP4-IgG in CSF: guidance for physicians. Neurol. Neuroimmunol. Neuroinflamm. 3, e231 (2016).
Gresa-Arribas, N. et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 13, 167–177 (2014).
Jezequel, J. et al. Cell- and single molecule-based methods to detect anti-N-methyl-D-aspartate receptor autoantibodies in patients with first-episode psychosis from the OPTiMiSE project. Biol. Psychiatry 82, 766–772 (2017).
Dujmovic, I. et al. Temporal dynamics of cerebrospinal fluid anti-aquaporin-4 antibodies in patients with neuromyelitis optica spectrum disorders. J. Neuroimmunol. 234, 124–130 (2011).
Jarius, S. et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J. Neuroinflammation 7, 52 (2010).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J. Neuroinflammation 13, 279 (2016).
Joubert, B. et al. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 73, 1115–1124 (2016).
Bien, C. G. et al. Anti-contactin-associated protein-2 encephalitis: relevance of antibody titres, presentation and outcome. Eur. J. Neurol. 24, 175–186 (2017).
Lucchinetti, C. F. et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125, 1450–1461 (2002).
Misu, T. et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 125, 815–827 (2013).
Roemer, S. F. et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 1194–1205 (2007).
Bien, C. G. et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135, 1622–1638 (2012).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Lehmann-Horn, K. et al. Intrathecal B-cell activation in LGI1 antibody encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 7, e669 (2020).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Lehmann-Horn, K., Wang, S.-Z., Sagan, S. A., Zamvil, S. S. & von Budingen, H.-C. B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight 1, e87234 (2016).
Bell, L., Lenhart, A., Rosenwald, A., Monoranu, C. M. & Berberich-Siebelt, F. Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front. Immunol. 10, 3090 (2019).
Chan, K. H., Lee, R., Lau, K. K. & Loong, F. Orbital ectopic lymphoid follicles with germinal centers in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. Front. Immunol. https://doi.org/10.3389/fimmu.2017.01947 (2018).
Armangue, T. et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 17, 760–772 (2018).
Liberman, A. C. et al. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front. Endocrinol. 9, 235 (2018).
Olnes, M. J. et al. Effects of systemically administered hydrocortisone on the human immunome. Sci. Rep. 6, 23002 (2016).
Lee, J. et al. Corticosteroid modulation of immunoglobulin expression and B-cell function in COPD. FASEB J. 30, 2014–2026 (2016).
Yan, S., Deng, X., Wang, Q., Sun, X. & Wei, W. Prednisone treatment inhibits the differentiation of B lymphocytes into plasma cells in MRL/MpSlac-lpr mice. Acta Pharmacol. Sin. 36, 1367–1376 (2015).
Franco, L. M. et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J. Exp. Med. 216, 384–406 (2019).
Cousins, D. J., McDonald, J. & Lee, T. H. Therapeutic approaches for control of transcription factors in allergic disease. J. Allergy Clin. Immunol. 121, 801–803 (2008).
Perugino, C. A. & Stone, J. H. Treatment of IgG4-related disease: current and future approaches. Z. Rheumatol. 75, 681–686 (2016).
Brito-Zeron, P. et al. Therapeutic approach to IgG4-related disease: a systematic review. Medicine 95, e4002 (2016).
Dubey, D. et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann. Neurol. 87, 313–323 (2020).
Konova, E., Atanasova, M., Stoykov, S., Velkova, A. & Shoenfeld, Y. Idiotypic and anti-idiotypic elastin autoantibodies: implications for IVIg and pregnancy loss. J. Autoimmun. 28, 46–54 (2007).
Kazatchkine, M. D. & Kaveri, S. V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 345, 747–755 (2001).
Sultan, Y., Kazatchkine, M. D., Maisonneuve, P. & Nydegger, U. E. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet 2, 765–768 (1984).
Guptill, J. T. et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity 49, 472–479 (2016).
Winters, J. L. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematol. Am. Soc. Hematol. Educ. Progr. 2012, 7–12 (2012).
Sun, X. et al. Clinical application of plasma exchange in pediatric anti-N-methyl-D-aspartate receptor encephalitis. Int. J. Clinial Exp. Med. 10, 11945–11952 (2017).
Graus, F., Abos, J., Roquer, J., Mazzara, R. & Pereira, A. Effect of plasmapheresis on serum and CSF autoantibody levels in CNS paraneoplastic syndromes. Neurology 40, 1621–1623 (1990).
Hanly, J. G., Hong, C., Zayed, E., Jones, J. V. & Jones, E. Immunomodulating effects of synchronised plasmapheresis and intravenous bolus cyclophosphamide in systemic lupus erythematosus. Lupus 4, 457–463 (1995).
Hofmann, K., Clauder, A.-K. & Manz, R. A. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 9, 835 (2018).
Lebrun, C. et al. Only follow-up of memory B cells helps monitor rituximab administration to patients with neuromyelitis optica spectrum disorders. Neurol. Ther. 7, 373–383 (2018).
Chamberlain, N. et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 126, 282–287 (2016).
Martin, F. & Chan, A. C. B cell immunobiology in disease: evolving concepts from the clinic. Annu. Rev. Immunol. 24, 467–496 (2006).
Monson, N. L., Cravens, P. D., Frohman, E. M., Hawker, K. & Racke, M. K. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch. Neurol. 62, 258–264 (2005).
Petereit, H. F. & Rubbert-Roth, A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult. Scler. 15, 189–192 (2009).
Bashford-Rogers, R. J. M. et al. Analysis of the B cell receptor repertoire in six immune-mediated diseases. Nature 574, 122–126 (2019).
Jiang, A. R., Fichtner, M. L., Hoehn, K. B. & Stathopoulos, P. Single-cell immune repertoire tracing identifies rituximab refractory B cells that emerge during relapse. JCI Insight https://doi.org/10.1172/jci.insight.136471 (2019).
Cupps, T. R., Edgar, L. C. & Fauci, A. S. Suppression of human B lymphocyte function by cyclophosphamide. J. Immunol. 128, 2453–2457 (1982).
Hoyer, B. F. et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199, 1577–1584 (2004).
Kessler, R. A., Mealy, M. A. & Levy, M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr. Treat. Options Neurol. 18, 2 (2016).
Stellmann, J.-P. et al. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J. Neurol. Neurosurg. Psychiatry 88, 639–647 (2017).
Clowse, M. E. B. et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res. 63, 1777–1781 (2011).
Nguyen, Q. N. et al. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 25, 433–444 (2019).
Zhang, C. et al. Safety and efficacy of bortezomib in patients with highly relapsing neuromyelitis optica spectrum disorder. JAMA Neurol. 74, 1010–1012 (2017).
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 14, 748–755 (2008).
Khodadadi, L. et al. Bortezomib plus continuous B cell depletion results in sustained plasma cell depletion and amelioration of lupus nephritis in NZB/W F1 mice. PLoS ONE 10, e0135081 (2015).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Patriquin, C. J. et al. Bortezomib in the treatment of refractory thrombotic thrombocytopenic purpura. Br. J. Haematol. 173, 779–785 (2016).
Rosenberg, A. S. et al. A role for plasma cell targeting agents in immune tolerance induction in autoimmune disease and antibody responses to therapeutic proteins. Clin. Immunol. 165, 55–59 (2016).
Kapoor, P., Ramakrishnan, V. & Rajkumar, S. V. Bortezomib combination therapy in multiple myeloma. Semin. Hematol. 49, 228–242 (2012).
Al-Diwani, A. et al. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry 6, 235–246 (2019).
Hughes, E. G. et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 30, 5866–5875 (2010).
Moscato, E. H. et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 76, 108–119 (2014).
Planaguma, J. et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 138, 94–109 (2015).
Ohkawa, T. et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J. Neurosci. 33, 18161–18174 (2013).
Sunwoo, J.-S. et al. Clinical manifestations of patients with CASPR2 antibodies. J. Neuroimmunol. 281, 17–22 (2015).
Ohkawa, T. et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J. Neurosci. 34, 8151–8163 (2014).
Petit-Pedrol, M. et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 13, 276–286 (2014).
Pettingill, P. et al. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology 84, 1233–1241 (2015).
Lancaster, E. et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 9, 67–76 (2010).
Dogan Onugoren, M. et al. Limbic encephalitis due to GABAB and AMPA receptor antibodies: a case series. J. Neurol. Neurosurg. Psychiatry 86, 965–972 (2015).
Nibber, A. et al. Pathogenic potential of antibodies to the GABAB receptor. Epilepsia Open 2, 355–359 (2017).
Collongues, N. et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 74, 736–742 (2010).
Pandit, L. et al. Demographic and clinical features of neuromyelitis optica: a review. Mult. Scler. 21, 845–853 (2015).
Hoftberger, R. et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult. Scler. 21, 866–874 (2015).
Waters, P. et al. Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol. 77, 82–93 (2020).
Mader, S. et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J. Neuroinflammation 8, 184 (2011).
Jarius, S. et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ‘pattern II multiple sclerosis’ and brain biopsy findings in a MOG-IgG-positive case. Mult. Scler. 22, 1541–1549 (2016).
Hoftberger, R. et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 139, 875–892 (2020).
Wardemann, H., Hammersen, J. & Nussenzweig, M. C. Human autoantibody silencing by immunoglobulin light chains. J. Exp. Med. 200, 191–199 (2004).
Silver, J. et al. Stochasticity enables BCR-independent germinal center initiation and antibody affinity maturation. J. Exp. Med. 215, 77–90 (2018).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 36, 569–577 (2015).
van Coevorden-Hameete, M. H. et al. The expanded clinical spectrum of anti-GABABR encephalitis and added value of KCTD16 autoantibodies. Brain 142, 1631–1643 (2019).
Spatola, M. et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology 88, 1012–1020 (2017).
Acknowledgements
B.S. is supported by the Association of British Neurologists via the Patrick Berthoud Charitable Trust. M.R. is supported by the Austrian Science Fund (FWF J4157-B30). S.R.I. is supported by the Wellcome Trust (104079/Z/14/Z), BMA Research Grants (Vera Down grant (2013) and Margaret Temple grant (2017)), Epilepsy Research UK (P1201), the Fulbright UK–US Commission (MS Society research award) and the National Institute for Health Research (NIHR), Oxford Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health. K.C.O’C. is supported by the National Institute of Allergy and Infectious Diseases (NIAID) under award numbers R01-AI114780 and R21-AI142198, by a Neuromuscular Disease Research program award from the Muscular Dystrophy Association (MDA) under award number MDA575198 and by the Guthy-Jackson Charitable Foundation.
Author information
Authors and Affiliations
Contributions
B.S., M.R. and S.R.I. researched data for the article and made substantial contributions to discussion of the content. All authors contributed to writing the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.J.M.B.-R. is a co-founder and consultant for Alchemab Therapeutics Ltd and has consulted for Imperial College London and VHSquared. S.R.I. and M.R. are co-inventors on a patent to improve the specificity of autoantibody detection. S.R.I. is a co-applicant and receives royalties on patent application WO/2010/046716 entitled ‘Neurological Autoimmune Disorders’. The patent has been licensed for the development of assays for LGI1 and other voltage-gated potassium channel (VGKC)-complex antibodies. S.R.I. has received research support from CSL Behring, ONO Pharma and UCB. B.S. declares no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks J. Honnorat, F. Leypoldt, S. Vernino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- B cell receptor
-
(BCR). An immunoglobulin molecule consisting of two paired identical heavy and light chains that forms a transmembrane receptor protein on the surface of B cells and signals to the B cell, largely via its interaction with its antigen or antigens.
- Anergy
-
A cell state in which B cells persist in the periphery but have limited responses to antigen; anergy is a mechanism that silences many self-reactive B cells.
- Receptor editing
-
A process in which autoreactive heavy or light chains of B cell receptors are exchanged, thereby changing the specificity of the antigen receptor and rescuing an autoreactive B cell receptor.
- B cell-activating factor
-
(BAFF). A cytokine that belongs to the tumour necrosis factor ligand family and is expressed in B cell lineage cells, acting as a potent B cell activator.
- Somatic hypermutation
-
The introduction of point mutations within the immunoglobulin variable regions in the presence of an antigen and T helper cells.
- Germinal centre
-
A site within a secondary lymphoid organ where the genes that encode immunoglobulins in B cells undergo somatic hypermutation, thereby sequentially increasing their affinity for the antigen; a process that typically requires B cells to undergo rounds of proliferation, differentiation and interactions with antigen and T cells.
- Unmutated common ancestors
-
(UCAs). Antibodies that are derived computationally and represent the most accurately matched germline B cell receptors from which the mutated antibodies were derived.
- Tertiary lymphoid structures
-
Ectopic lymphoid-like tissues with features of secondary lymphoid organs, such as segregated T and B cell zones, mutational activity, follicular dendritic cell networks and high endothelial venules.
- T follicular helper cells
-
Germinal centre resident CD4+ T follicular helper cells that provide potent survival and proliferative signals for B cells.
- Human leukocyte antigen class II
-
(HLA class II). Proteins expressed on the surface of antigen-presenting cells and, if loaded with a cognate peptide, can activate antigen-specific CD4+ T cells. Also known as major histocompatibility complex class II.
- Glutamic acid decarboxylase
-
(GAD). A cytoplasmic antigen, autoantibodies to which can be found at high levels in patients with neurological syndromes such as stiff-person syndrome, cerebellar ataxia, limbic encephalitis and forms of epilepsy and diabetes.
- Antibody index
-
A calculation used to assess intrathecal IgG synthesis by comparing the ratio of specific autoantibody levels to total levels of IgG in serum and CSF (antibody index = Qspec/QIgG, where Qspec = CSF/serum quotient for specific IgG, and QIgG = CSF/serum quotient for total IgG; values >4 are taken as evidence for intrathecal autoantigen-specific IgG synthesis).
Rights and permissions
About this article
Cite this article
Sun, B., Ramberger, M., O’Connor, K.C. et al. The B cell immunobiology that underlies CNS autoantibody-mediated diseases. Nat Rev Neurol 16, 481–492 (2020). https://doi.org/10.1038/s41582-020-0381-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0381-z
This article is cited by
-
Origins and immunopathogenesis of autoimmune central nervous system disorders
Nature Reviews Neurology (2023)
-
Autoimmune encephalitis: recent clinical and biological advances
Journal of Neurology (2023)
-
Anti-IgLON5 antibodies cause progressive behavioral and neuropathological changes in mice
Journal of Neuroinflammation (2022)
-
Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson´s disease
npj Parkinson's Disease (2022)
-
Treatment Options in Refractory Autoimmune Encephalitis
CNS Drugs (2022)