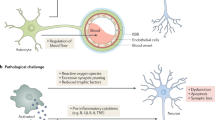
Abstract
Frontotemporal dementia (FTD) refers to a group of progressive neurodegenerative disorders with different pathological signatures, genetic variability and complex disease mechanisms, for which no effective treatments exist. Despite advances in understanding the underlying pathology of FTD, sensitive and specific fluid biomarkers for this disease are lacking. As in other types of dementia, mounting evidence suggests that neuroinflammation is involved in the progression of FTD, including cortical inflammation, microglial activation, astrogliosis and differential expression of inflammation-related proteins in the periphery. Furthermore, an overlap between FTD and autoimmune disease has been identified. The most substantial evidence, however, comes from genetic studies, and several FTD-related genes are also implicated in neuroinflammation. This Review discusses specific evidence of neuroinflammatory mechanisms in FTD and describes how advances in our understanding of these mechanisms, in FTD as well as in other neurodegenerative diseases, might facilitate the development and implementation of diagnostic tools and disease-modifying treatments for FTD.
Key points
-
Neuroinflammation is a major contributor to the pathogenic process in frontotemporal dementia (FTD).
-
Exploration of neuroinflammatory pathways, immune-mediated mechanisms and the use of immunomodulation as a disease-modification strategy are promising research directions in this setting.
-
Growing understanding of the complexity of microglial subpopulations provides an opportunity to explore the phenotypic landscape of microglia-driven neuroinflammation in FTD.
-
Replication and validation of previous studies in human FTD using appropriate controls, independent cohorts and quantitative methods is pivotal for the identification of new treatment targets and drug and biomarker candidates.
-
More knowledge is required on the context (cell type, timing of assessment and disease stage) of FTD-related gene effects on neuroinflammation.
-
A combination of clinical biomarker discovery and studies of patients with FTD carrying mutations that target a specific protein or underlying pathology will be essential in future investigations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hodges, J. R. & Miller, B. The classification, genetics and neuropathology of frontotemporal dementia. Introduction to the special topic papers: part I. Neurocase 7, 31–35 (2001).
McKhann, G. M. et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch. Neurol. 58, 1803–1809 (2001).
Burrell, J. R. et al. The frontotemporal dementia–motor neuron disease continuum. Lancet 388, 919–931 (2016).
Hogan, D. B. et al. The prevalence and incidence of frontotemporal dementia: a systematic review. Can. J. Neurol. Sci. 43 (Suppl. 1), 96–109 (2016).
Coyle-Gilchrist, I. T. et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86, 1736–1743 (2016).
Ratnavalli, E., Brayne, C., Dawson, K. & Hodges, J. R. The prevalence of frontotemporal dementia. Neurology 58, 1615–1621 (2002).
Hodges, J. R. & Piguet, O. Progress and challenges in frontotemporal dementia research: a 20-year review. J. Alzheimers Dis. 62, 1467–1480 (2018).
Ahmed, R. M. et al. Mouse models of frontotemporal dementia: a comparison of phenotypes with clinical symptomatology. Neurosci. Biobehav. Rev. 74, 126–138 (2017).
Ittner, L. M. et al. FTD and ALS — translating mouse studies into clinical trials. Nat. Rev. Neurol. 11, 360–366 (2015).
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21, 1370–1379 (2018).
Ferrari, R. et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 13, 686–699 (2014).
Broce, I. et al. Immune-related genetic enrichment in frontotemporal dementia: an analysis of genome-wide association studies. PLOS Med. 15, e1002487 (2018).
Miller, Z. A. et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J. Neurol. Neurosurg. Psychiatry 84, 956–962 (2013).
Miller, Z. A. et al. Increased prevalence of autoimmune disease within C9 and FTD/MND cohorts: completing the picture. Neurol. Neuroimmunol. Neuroinflamm. 3, e301 (2016).
Cerami, C., Iaccarino, L. & Perani, D. Molecular imaging of neuroinflammation in neurodegenerative dementias: the role of in vivo PET imaging. Int. J. Mol. Sci. 18, E993 (2017).
Wyss-Coray, T. & Mucke, L. Inflammation in neurodegenerative disease — a double-edged sword. Neuron 35, 419–432 (2002).
Pasqualetti, G., Brooks, D. J. & Edison, P. The role of neuroinflammation in dementias. Curr. Neurol. Neurosci. Rep. 15, 17 (2015).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Mrak, R. E. & Griffin, W. S. Common inflammatory mechanisms in Lewy body disease and Alzheimer disease. J. Neuropathol. Exp. Neurol. 66, 683–686 (2007).
Imamura, K. et al. Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 109, 141–150 (2005).
Morales, I., Guzman-Martinez, L., Cerda-Troncoso, C., Farias, G. A. & Maccioni, R. B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 8, 112 (2014).
Sochocka, M., Diniz, B. S. & Leszek, J. Inflammatory response in the CNS: friend or foe? Mol. Neurobiol. 54, 8071–8089 (2017).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Gratwicke, J., Jahanshahi, M. & Foltynie, T. Parkinson’s disease dementia: a neural networks perspective. Brain 138, 1454–1476 (2015).
McGeer, P. L. & McGeer, E. G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26, 459–470 (2002).
Chitnis, T. & Weiner, H. L. CNS inflammation and neurodegeneration. J. Clin. Invest. 127, 3577–3587 (2017).
Ransohoff, R. M. How neuroinflammation contributes to neurodegeneration. Science 353, 777–783 (2016).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Rubartelli, A. & Lotze, M. T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 (2007).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139 (Suppl. 2), 136–153 (2016).
Schwartz, M. & Baruch, K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 33, 7–22 (2014).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Ramesh, G., MacLean, A. G. & Philipp, M. T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013, 480739 (2013).
González, H., Elgueta, D., Montoya, A. & Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 274, 1–13 (2014).
Meeter, L. H., Kaat, L. D., Rohrer, J. D. & van Swieten, J. C. Imaging and fluid biomarkers in frontotemporal dementia. Nat. Rev. Neurol. 13, 406–419 (2017).
Skaper, S. D., Giusti, P. & Facci, L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 26, 3103–3117 (2012).
Carrano, A. et al. Neuroinflammation and blood–brain barrier changes in capillary amyloid angiopathy. Neurodegener. Dis. 10, 329–331 (2012).
Bowman, G. L. et al. Blood–brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 14, 1640–1650 (2018).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Zlokovic, B. V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Cagnin, A., Rossor, M., Sampson, E. L., Mackinnon, T. & Banati, R. B. In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 56, 894–897 (2004).
Lant, S. B. et al. Patterns of microglial cell activation in frontotemporal lobar degeneration. Neuropathol. Appl. Neurobiol. 40, 686–696 (2014).
Taipa, R. et al. Patterns of microglial cell activation in Alzheimer disease and frontotemporal lobar degeneration. Neurodegener. Dis. 17, 145–154 (2017).
Kersaitis, C., Halliday, G. M. & Kril, J. J. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 108, 515–523 (2004).
Arnold, S. E., Han, L. Y., Clark, C. M., Grossman, M. & Trojanowski, J. Q. Quantitative neurohistological features of frontotemporal degeneration. Neurobiol. Aging 21, 913–919 (2000).
Alberici, A. et al. Autoimmunity and frontotemporal dementia. Curr. Alzheimer Res. 15, 602–609 (2018).
Bohlen, C. J. et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Spiller, K. J. et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat. Neurosci. 21, 329–340 (2018).
Sandiego, C. M. et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl Acad. Sci. USA 112, 12468–12473 (2015).
Kim, E. J. & Yu, S. W. Translocator protein 18 kDa (TSPO): old dogma, new mice, new structure, and new questions for neuroprotection. Neural Regen. Res. 10, 878–880 (2015).
Notter, T. et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatry 23, 323–334 (2018).
Narayan, N. et al. The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLOS ONE 12, e0185767 (2017).
Beckers, L. et al. Increased expression of translocator protein (TSPO) marks pro-inflammatory microglia but does not predict neurodegeneration. Mol. Imaging Biol. 20, 94–102 (2018).
Owen, D. R. et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J. Cereb. Blood Flow Metab. 37, 2679–2690 (2017).
Woollacott, I. O. C. et al. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia. Neurocase 24, 166–174 (2018).
Bellucci, A., Bugiani, O., Ghetti, B. & Spillantini, M. G. Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation. Neurodegener. Dis. 8, 221–229 (2011).
Brettschneider, J. et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLOS ONE 7, e39216 (2012).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, e1738–e1746 (2016).
Satoh, J. et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology 36, 39–49 (2016).
Umoh, M. E. et al. A proteomic network approach across the ALS–FTD disease spectrum resolves clinical phenotypes and genetic vulnerability in human brain. EMBO Mol. Med. 10, 48–62 (2018).
Friedman, B. A. et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 22, 832–847 (2018).
Schwartz, M., Butovsky, O. & Kipnis, J. Does inflammation in an autoimmune disease differ from inflammation in neurodegenerative diseases? Possible implications for therapy. J. Neuroimmune Pharmacol. 1, 4–10 (2006).
de Haan, P., Klein, H. C. & ‘t Hart, B. A. Autoimmune aspects of neurodegenerative and psychiatric diseases: a template for innovative therapy. Front. Psychiatry 8, 46 (2017).
Kortvelyessy, P. et al. Biomarkers of neurodegeneration in autoimmune-mediated encephalitis. Front. Neurol. 9, 668 (2018).
Graus, F. et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 71, 930–936 (2008).
Sabater, L. et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 13, 575–586 (2014).
Borroni, B. et al. Autoimmune frontotemporal dementia: a new nosological entity? Alzheimer Dis. Assoc. Disord. 31, 259–262 (2017).
Borroni, B. et al. Anti-AMPA GluA3 antibodies in frontotemporal dementia: a new molecular target. Sci. Rep. 7, 6723 (2017).
Younes, K., Lepow, L. A., Estrada, C. & Schulz, P. E. Auto-antibodies against P/Q− and N-type voltage-dependent calcium channels mimicking frontotemporal dementia. SAGE Open Med. Case Rep. 6, 205031317750928X (2018).
Cavazzana, I. et al. Antinuclear antibodies in frontotemporal dementia: the tip’s of autoimmunity iceberg? J. Neuroimmunol. 325, 61–63 (2018).
Yamamoto, Y. et al. Increased serum GP88 (progranulin) concentrations in rheumatoid arthritis. Inflammation 37, 1806–1813 (2014).
Chen, J. et al. Serum progranulin irrelated with Breg cell levels, but elevated in RA patients, reflecting high disease activity. Rheumatol. Int. 36, 359–364 (2016).
Zhang, N., Yang, N., Chen, Q., Qiu, F. & Li, X. Upregulated expression level of the growth factor, progranulin, is associated with the development of primary Sjögren’s syndrome. Exp. Ther. Med. 8, 1643–1647 (2014).
Huang, K. et al. Progranulin is preferentially expressed in patients with psoriasis vulgaris and protects mice from psoriasis-like skin inflammation. Immunology 145, 279–287 (2015).
Jian, J., Li, G., Hettinghouse, A. & Liu, C. Progranulin: a key player in autoimmune diseases. Cytokine 101, 48–55 (2018).
Pottier, C. et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 137, 879–899 (2019).
Piguet, O. Neurodegenerative disease: frontotemporal dementia — time to target inflammation? Nat. Rev. Neurol. 9, 304–305 (2013).
Lan, Y., Sullivan, P. M. & Hu, F. SMCR8 negatively regulates AKT and MTORC1 signaling to modulate lysosome biogenesis and tissue homeostasis. Autophagy 15, 871–885 (2019).
Katisko, K. et al. Prevalence of immunological diseases in a Finnish frontotemporal lobar degeneration cohort with the C9orf72 repeat expansion carriers and non-carriers. J. Neuroimmunol. 321, 29–35 (2018).
Burrell, J. R. & Hodges, J. R. Could immunological mechanisms trigger neurodegeneration in frontotemporal dementia? J. Neurol. Neurosurg. Psychiatry 84, 946 (2013).
Pottier, C., Ravenscroft, T. A., Sanchez-Contreras, M. & Rademakers, R. Genetics of FTLD: overview and what else we can expect from genetic studies. J. Neurochem. 138 (Suppl. 1), 32–53 (2016).
Cirulli, E. T. et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347, 1436–1441 (2015).
Freischmidt, A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 (2015).
Su, W. H. et al. The rs75932628 and rs2234253 polymorphisms of the TREM2 gene were associated with susceptibility to frontotemporal lobar degeneration in Caucasian populations. Ann. Hum. Genet. 82, 177–185 (2018).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Smith, K. R. et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 90, 1102–1107 (2012).
Almeida, M. R. et al. Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol. Aging 41, 200.e1–200.e5 (2016).
Castaneda, J. A., Lim, M. J., Cooper, J. D. & Pearce, D. A. Immune system irregularities in lysosomal storage disorders. Acta Neuropathol. 115, 159–174 (2008).
Van Damme, P. et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J. Cell Biol. 181, 37–41 (2008).
Tapia, L. et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J. Neurosci. 31, 11126–11132 (2011).
Petkau, T. L. et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol. Dis. 45, 711–722 (2012).
Bateman, A., Belcourt, D., Bennett, H., Lazure, C. & Solomon, S. Granulins, a novel class of peptide from leukocytes. Biochem. Biophys. Res. Commun. 173, 1161–1168 (1990).
Moisse, K. et al. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: implications for TDP-43 in the physiological response to neuronal injury. Brain Res. 1249, 202–211 (2009).
Naphade, S. B. et al. Progranulin expression is upregulated after spinal contusion in mice. Acta Neuropathol. 119, 123–133 (2010).
Tanaka, Y., Matsuwaki, T., Yamanouchi, K. & Nishihara, M. Exacerbated inflammatory responses related to activated microglia after traumatic brain injury in progranulin-deficient mice. Neuroscience 231, 49–60 (2013).
Martens, L. H. et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Invest. 122, 3955–3959 (2012).
Yin, F. et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 207, 117–128 (2010).
Altmann, C. et al. Progranulin promotes peripheral nerve regeneration and reinnervation: role of notch signaling. Mol. Neurodegener. 11, 69 (2016).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Filiano, A. J. et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J. Neurosci. 33, 5352–5361 (2013).
Bossu, P. et al. Loss of function mutations in the progranulin gene are related to pro-inflammatory cytokine dysregulation in frontotemporal lobar degeneration patients. J. Neuroinflamm. 8, 65 (2011).
Galimberti, D. et al. Inflammatory molecules in frontotemporal dementia: cerebrospinal fluid signature of progranulin mutation carriers. Brain Behav. Immun. 49, 182–187 (2015).
Milanesi, E. et al. Molecular signature of disease onset in granulin mutation carriers: a gene expression analysis study. Neurobiol. Aging 34, 1837–1845 (2013).
Zhu, J. et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111, 867–878 (2002).
Pickford, F. et al. Progranulin is a chemoattractant for microglia and stimulates their endocytic activity. Am. J. Pathol. 178, 284–295 (2011).
Suh, H. S., Choi, N., Tarassishin, L. & Lee, S. C. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLOS ONE 7, e35115 (2012).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Moens, T. G., Partridge, L. & Isaacs, A. M. Genetic models of C9orf72: what is toxic? Curr. Opin. Genet. Dev. 44, 92–101 (2017).
Therrien, M., Rouleau, G. A., Dion, P. A. & Parker, J. A. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLOS ONE 8, e83450 (2013).
Ciura, S. et al. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann. Neurol. 74, 180–187 (2013).
Atanasio, A. et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci. Rep. 6, 23204 (2016).
O’Rourke, J. G. et al. C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016).
Burberry, A. et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci. Transl Med. 8, 347ra93 (2016).
Sudria-Lopez, E. et al. Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects. Acta Neuropathol. 132, 145–147 (2016).
Schludi, M. H. et al. Spinal poly-GA inclusions in a C9orf72 mouse model trigger motor deficits and inflammation without neuron loss. Acta Neuropathol. 134, 241–254 (2017).
Chew, J. et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science 348, 1151–1154 (2015).
Jiang, J. et al. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90, 535–550 (2016).
Liu, Y. et al. C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron 90, 521–534 (2016).
Zhang, Y. J. et al. C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat. Neurosci. 19, 668–677 (2016).
Ahmad, L., Zhang, S. Y., Casanova, J. L. & Sancho-Shimizu, V. Human TBK1: a gatekeeper of neuroinflammation. Trends Mol. Med. 22, 511–527 (2016).
Fitzgerald, K. A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Bonnard, M. et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19, 4976–4985 (2000).
Oakes, J. A., Davies, M. C. & Collins, M. O. TBK1: a new player in ALS linking autophagy and neuroinflammation. Mol. Brain 10, 5 (2017).
Fingert, J. H. et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum. Mol. Genet. 20, 2482–2494 (2011).
Ritch, R. et al. TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol. 132, 544–548 (2014).
Awadalla, M. S. et al. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. Am. J. Ophthalmol. 159, 124–130 (2015).
Herman, M. et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 209, 1567–1582 (2012).
Yu, J. et al. Regulation of T cell activation and migration by the kinase TBK1 during neuroinflammation. Nat. Commun. 6, 6074 (2015).
Maruyama, H. et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226 (2010).
Fecto, F. et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 68, 1440–1446 (2011).
Rubino, E. et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 79, 1556–1562 (2012).
Pottier, C. et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 130, 77–92 (2015).
Wild, P. et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011).
Pilli, M. et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37, 223–234 (2012).
Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 3, 445–453 (2003).
Sessa, G. et al. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur. J. Neurosci. 20, 2617–2628 (2004).
Jay, T. R., von Saucken, V. E. & Landreth, G. E. TREM2 in neurodegenerative diseases. Mol. Neurodegener. 12, 56 (2017).
Yeh, F. L., Hansen, D. V. & Sheng, M. TREM2, microglia, and neurodegenerative diseases. Trends Mol. Med. 23, 512–533 (2017).
Sieber, M. W. et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLOS ONE 8, e52982 (2013).
Kawabori, M. et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 35, 3384–3396 (2015).
Saber, M., Kokiko-Cochran, O., Puntambekar, S. S., Lathia, J. D. & Lamb, B. T. Triggering receptor expressed on myeloid cells 2 deficiency alters acute macrophage distribution and improves recovery after traumatic brain injury. J. Neurotrauma 34, 423–435 (2017).
Hsieh, C. L. et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J. Neurochem. 109, 1144–1156 (2009).
Poliani, P. L. et al. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 (2015).
Mazaheri, F. et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 18, 1186–1198 (2017).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015).
Paloneva, J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 (2002).
Verloes, A. et al. Nasu–Hakola syndrome: polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J. Med. Genet. 34, 753–757 (1997).
Satoh, J. I., Kino, Y., Yanaizu, M. & Saito, Y. Alzheimer’s disease pathology in Nasu–Hakola disease brains. Intractable Rare Dis. Res. 7, 32–36 (2018).
Gratuze, M., Leyns, C. E. G. & Holtzman, D. M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 13, 66 (2018).
Guerreiro, R. J. et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 70, 78–84 (2013).
Giraldo, M. et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol. Aging 34, 2077.e11–2077.e18 (2013).
Le Ber, I. et al. Homozygous TREM2 mutation in a family with atypical frontotemporal dementia. Neurobiol. Aging 35, 2419.e23–2419.e25 (2014).
Lu, Y., Liu, W. & Wang, X. TREM2 variants and risk of Alzheimer’s disease: a meta-analysis. Neurol. Sci. 36, 1881–1888 (2015).
Jiang, T. et al. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiol. Aging 36, 3176–3186 (2015).
Leyns, C. E. G. et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl Acad. Sci. USA 114, 11524–11529 (2017).
Kleinberger, G. et al. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J. 36, 1837–1853 (2017).
Zetterberg, H., van Swieten, J. C., Boxer, A. L. & Rohrer, J. D. Review: fluid biomarkers for frontotemporal dementias. Neuropathol. Appl. Neurobiol. 45, 81–87 (2019).
Borroni, B. et al. Biological, neuroimaging, and neurophysiological markers in frontotemporal dementia: three faces of the same coin. J. Alzheimers Dis. 62, 1113–1123 (2018).
Rohrer, J. D. et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 14, 253–262 (2015).
Ghidoni, R., Benussi, L., Glionna, M., Franzoni, M. & Binetti, G. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology 71, 1235–1239 (2008).
Finch, N. et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132, 583–591 (2009).
Schofield, E. C. et al. Low serum progranulin predicts the presence of mutations: a prospective study. J. Alzheimers Dis. 22, 981–984 (2010).
Galimberti, D. et al. Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: results from the GENFI study. Neurobiol. Aging 62, 245.e9–245.e12 (2018).
Kortvelyessy, P., Heinze, H. J., Prudlo, J. & Bittner, D. CSF biomarkers of neurodegeneration in progressive non-fluent aphasia and other forms of frontotemporal dementia: clues for pathomechanisms? Front. Neurol. 9, 504 (2018).
Galimberti, D., Fenoglio, C. & Scarpini, E. Progranulin as a therapeutic target for dementia. Expert Opin. Ther. Targets 22, 579–585 (2018).
Alberici, A. et al. Results from a pilot study on amiodarone administration in monogenic frontotemporal dementia with granulin mutation. Neurol. Sci. 35, 1215–1219 (2014).
Holler, C. J. et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: a novel therapeutic lead to treat frontotemporal dementia. Mol. Neurodegener. 11, 46 (2016).
Lee, W. C. et al. Targeted manipulation of the sortilin–progranulin axis rescues progranulin haploinsufficiency. Hum. Mol. Genet. 23, 1467–1478 (2014).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2011).
Woollacott, I. O. C. et al. Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res. Ther. 10, 79 (2018).
Dembic, Z. in The Cytokines of the Immune System 57–98 (Academic Press, 2015).
Hu, W. T. et al. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology 75, 2079–2086 (2010).
Sjögren, M., Folkesson, S., Blennow, K. & Tarkowski, E. Increased intrathecal inflammatory activity in frontotemporal dementia: pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 75, 1107–1111 (2004).
Gibbons, L. et al. Plasma levels of progranulin and interleukin-6 in frontotemporal lobar degeneration. Neurobiol. Aging 36, 16030–16034 (2015).
Nilsson, C., Landqvist Waldo, M., Nilsson, K., Santillo, A. & Vestberg, S. Age-related incidence and family history in frontotemporal dementia: data from the Swedish Dementia Registry. PLOS ONE 9, e94901 (2014).
Reitz, C., Brayne, C. & Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 7, 137–152 (2011).
Wehrspaun, C. C., Haerty, W. & Ponting, C. P. Microglia recapitulate a hematopoietic master regulator network in the aging human frontal cortex. Neurobiol. Aging 36, 2443.e9–2443.e20 (2015).
Cardona, A. E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006).
Lukiw, W. J. Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem. Res. 29, 1287–1297 (2004).
Sheng, J. G., Mrak, R. E. & Griffin, W. S. Enlarged and phagocytic, but not primed, interleukin-1α-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 95, 229–234 (1998).
Werry, E. L., Enjeti, S., Halliday, G. M., Sachdev, P. S. & Double, K. L. Effect of age on proliferation-regulating factors in human adult neurogenic regions. J. Neurochem. 115, 956–964 (2010).
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004).
Rawji, K. S. et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661 (2016).
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Reu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Posfai, B., Cserep, C., Orsolits, B. & Denes, A. New insights into microglia–neuron interactions: a neuron’s perspective. Neuroscience 405, 103–117 (2018).
Bu, X. L. et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 22, 1519–1525 (2015).
Lauridsen, J. K. et al. High BMI levels associate with reduced mRNA expression of IL10 and increased mRNA expression of iNOS (NOS2) in human frontal cortex. Transl Psychiatry 7, e1044 (2017).
Rosso, S. M. et al. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case–control study. J. Neurol. Neurosurg. Psychiatry 74, 1574–1576 (2003).
Wang, H. K. et al. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience 300, 94–103 (2015).
Soreq, L. et al. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18, 557–570 (2017).
Olah, M. et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 9, 539 (2018).
Cribbs, D. H. et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflamm. 9, 179 (2012).
Streit, W. J., Sammons, N. W., Kuhns, A. J. & Sparks, D. L. Dystrophic microglia in the aging human brain. Glia 45, 208–212 (2004).
Tay, T. L. et al. Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 10, 421 (2017).
Streit, W. J., Braak, H., Xue, Q. S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 118, 475–485 (2009).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Chinta, S. J. et al. Cellular senescence and the aging brain. Exp. Gerontol. 68, 3–7 (2015).
Jyothi, H. J. et al. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol. Aging 36, 3321–3333 (2015).
David, J. P. et al. Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci. Lett. 235, 53–56 (1997).
Nichols, N. R., Day, J. R., Laping, N. J., Johnson, S. A. & Finch, C. E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 14, 421–429 (1993).
Campuzano, O., Castillo-Ruiz, M. M., Acarin, L., Castellano, B. & Gonzalez, B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J. Neurosci. Res. 87, 2484–2497 (2009).
Krabbe, G. et al. Microglial NFκB–TNFα hyperactivation induces obsessive-compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia. Proc. Natl Acad. Sci. USA 114, 5029–5034 (2017).
Arrant, A. E., Onyilo, V. C., Unger, D. E. & Roberson, E. D. Progranulin gene therapy improves lysosomal dysfunction and microglial pathology associated with frontotemporal dementia and neuronal ceroid lipofuscinosis. J. Neurosci. 38, 2341–2358 (2018).
Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M. & Spillantini, M. G. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939–1948 (2018).
Geloso, M. C. et al. The dual role of microglia in ALS: mechanisms and therapeutic approaches. Front. Aging Neurosci. 9, 242 (2017).
Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Santos, R. R. et al. Reduced frequency of T lymphocytes expressing CTLA-4 in frontotemporal dementia compared to Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 1–5 (2014).
Galimberti, D. et al. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer’s disease and frontotemporal lobar degeneration. J. Neurol. 255, 539–544 (2008).
Galimberti, D. et al. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology 66, 146–147 (2006).
Rentzos, M. et al. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J. Neurol. Sci. 249, 110–114 (2006).
Torres, K. C. et al. Decreased expression of CCL3 in monocytes and CCR5 in lymphocytes from frontotemporal dementia as compared with Alzheimer’s disease patients. J. Neuropsychiatry Clin. Neurosci. 24, 2 (2012).
Galimberti, D. et al. MCP-1 A-2518G polymorphism: effect on susceptibility for frontotemporal lobar degeneration and on cerebrospinal fluid MCP-1 levels. J. Alzheimers Dis. 17, 125–133 (2009).
Busse, M. et al. Alterations in the peripheral immune system in dementia. J. Alzheimers Dis. 58, 1303–1313 (2017).
Teunissen, C. E. et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement. 2, 86–94 (2016).
Kokiko-Cochran, O. N. et al. Traumatic brain injury in hTau model mice: enhanced acute macrophage response and altered long-term recovery. J. Neurotrauma 35, 73–84 (2018).
Clayton, E. L. et al. Early microgliosis precedes neuronal loss and behavioural impairment in mice with a frontotemporal dementia-causing CHMP2B mutation. Hum. Mol. Genet. 26, 873–887 (2017).
Dec, E. et al. Cytokine profiling in patients with VCP-associated disease. Clin. Transl Sci. 7, 29–32 (2014).
Li, J.-M. M., Wu, H., Zhang, W., Blackburn, M. R. & Jin, J. The p97–UFD1L–NPL4 protein complex mediates cytokine-induced IκBα proteolysis. Mol. Cell. Biol. 34, 335–347 (2014).
Nalbandian, A. et al. Activation of the NLRP3 inflammasome is associated with valosin-containing protein myopathy. Inflammation 40, 21–41 (2017).
Sanz, L., Sanchez, P., Lallena, M. J., Diaz-Meco, M. T. & Moscat, J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J. 18, 3044–3053 (1999).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Munitic, I. et al. Optineurin insufficiency impairs IRF3 but not NF-κB activation in immune cells. J. Immunol. 191, 6231–6240 (2013).
Meena, N. P. et al. The TBK1-binding domain of optineurin promotes type I interferon responses. FEBS Lett. 590, 1498–1508 (2016).
Chew, T. S. et al. Optineurin deficiency in mice contributes to impaired cytokine secretion and neutrophil recruitment in bacteria-driven colitis. Dis. Model. Mech. 8, 817–829 (2015).
Wils, H. et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc. Natl Acad. Sci. USA 107, 3858–3863 (2010).
Paolicelli, R. C. et al. TDP-43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron 95, 297–308 (2017).
Rhinn, H. & Abeliovich, A. Differential aging analysis in human cerebral cortex identifies variants in TMEM106B and GRN that regulate aging phenotypes. Cell Syst. 4, 404–415 (2017).
Acknowledgements
The authors’ research is supported by the National Health and Medical Research Council of Australia (NHMRC) grant numbers 1132524, 1095127, 1140708 and 1081916 to G.M.H., J.J.K., O.P., M.C.K., J.R.H., L.M.I. and M.K. and by the University of Sydney. C.D.-S. is an NHMRC Boosting Dementia Research Leadership Fellow (NHMRC grant number 1138223); G.M.H. is an NHMRC Senior Principal Research Fellow (NHMRC grant number 1079679); L.M.I. is an NHMRC Principal Research Fellow (NHMRC grant number 1136241); O.P. is an NHMRC Senior Research Fellow (NHMRC grant number APP1103258); C.T.L. is an NHMRC Dementia Fellow (NHMRC grant number 1107657). The authors thank H. Cartwright from the Brain and Mind Centre, University of Sydney, Australia, for her assistance with figure design and creation.
Author information
Authors and Affiliations
Contributions
J.J.K., F.B., E.L.W., C.D.-S. and C.T.L. researched data for the manuscript. F.B., E.L.W. and C.D.-S. wrote the first draft. J.J.K., F.B., E.L.W., C.D.-S. and M.K. contributed substantially to discussions of the article content. J.J.K., O.P., L.M.I., G.M.H., J.R.H., M.C.K., C.T.L. and M.K. contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.R.H. declares that he is an editorial board member of the journals Cognitive Neuropsychiatry, Aphasiology, Cognitive Neuropsychology, Nature Reviews Neurology, Journal of Alzheimer’s Disease, Acta Neuropsychologica, ALS-FTD Journal, Neurology and Clinical Neuroscience, Dementia Neurospsychologia; and has received research support from the National Health and Medical Research Council of Australia (NHMRC), the Australian Research Council and institutional support from the University of Sydney. M.C.K. declares that he serves as Editor-in-Chief of the Journal of Neurology, Neurosurgery and Psychiatry. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks M. Heneka, D. Galimberti, and other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bright, F., Werry, E.L., Dobson-Stone, C. et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 15, 540–555 (2019). https://doi.org/10.1038/s41582-019-0231-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-019-0231-z
This article is cited by
-
Vascular dysfunction in sporadic bvFTD: white matter hyperintensity and peripheral vascular biomarkers
Alzheimer's Research & Therapy (2024)
-
Preclinical translational platform of neuroinflammatory disease biology relevant to neurodegenerative disease
Journal of Neuroinflammation (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Evidenced-based biological prevention and intervention strategies of dementia: a narrative review
Current Psychology (2024)
-
VCP Inhibition Augments NLRP3 Inflammasome Activation
Inflammation (2024)