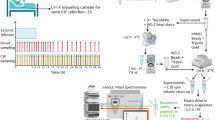
Abstract
Alzheimer disease (AD) is one of several neurodegenerative diseases characterized by dysregulation, misfolding and accumulation of specific proteins in the CNS. The stable isotope labelling kinetics (SILK) technique is based on generating amino acids labelled with naturally occurring stable (that is, nonradioactive) isotopes of carbon and/or nitrogen. These labelled amino acids can then be incorporated into proteins, enabling rates of protein production and clearance to be determined in vivo and in vitro without the use of radioactive or chemical labels. Over the past decade, SILK studies have been used to determine the turnover of key pathogenic proteins amyloid-β (Aβ), tau and superoxide dismutase 1 (SOD1) in the cerebrospinal fluid of healthy individuals, patients with AD and those with other neurodegenerative diseases. These studies led to the identification of several factors that alter the production and/or clearance of these proteins, including age, sleep and disease-causing genetic mutations. SILK studies have also been used to measure Aβ turnover in blood and within brain tissue. SILK studies offer the potential to elucidate the mechanisms underlying various neurodegenerative disease mechanisms, including neuroinflammation and synaptic dysfunction, and to demonstrate target engagement of novel disease-modifying therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilkinson, D. J. Historical and contemporary stable isotope tracer approaches to studying mammalian protein metabolism. Mass Spectrom. Rev. 37, 57–80 (2018).
Gregg, C. T. et al. Substantial replacement of mammalian body carbon with carbon-13. Life Sci. 13, 775–782 (1973).
Haass, C. & Selkoe, D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75, 1039–1042 (1993).
Xie, L. L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Roberts, K. F. et al. Amyloid-β efflux from the central nervous system into the plasma. Ann. Neurol. 76, 837–844 (2014).
Eckman, E. A. & Eckman, C. B. Aβ-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. 33, 1101–1105 (2005).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Patterson, B. W. et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann. Neurol. 78, 439–453 (2015).
Wildsmith, K. R. et al. In vivo human apolipoprotein E isoform fractional turnover rates in the CNS. PLOS ONE 7, e38013 (2012).
Potter, R. et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl Med. 5, 189ra77 (2013).
Bateman, R. J. et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 66, 48–54 (2009).
Lucey, B. P. et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann. Neurol. 83, 197–204 (2018).
Bateman, R. J. et al. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 12, 856–861 (2006).
Mawuenyega, K. G. et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330, 1774 (2010).
Dobrowolska, J. A. et al. CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition. J. Neurosci. 34, 8336–8346 (2014).
Cook, J. J. et al. Acute γ-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-β production to alternative APP fragments without amyloid-β rebound. J. Neurosci. 30, 6743–6750 (2010).
Ovod, V. et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 13, 841–849 (2017).
Alzforum. Finally a blood test for Alzheimer’s? Series — Alzheimer’s Association International Conference 2017: part 1 of 16. Alzforum https://www.alzforum.org/news/conference-coverage/finally-blood-test-alzheimers (2017).
Kang, J. E. et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009).
Huang, Y. F. et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch. Neurol. 69, 51–58 (2012).
Lucey, B. P. & Bateman, R. J. Amyloid-β diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol. Aging 35, S29–S34 (2014).
Wildburger, N. C. et al. Amyloid-β plaques in clinical Alzheimer’s disease brain incorporate stable isotope tracer in vivo and exhibit nanoscale heterogeneity. Front. Neurol. 9, 169 (2018).
Castellano, J. M. et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl Med. 3, 89ra57 (2011).
Basak, J. M. et al. Measurement of apolipoprotein E and amyloid β clearance rates in the mouse brain using bolus stable isotope labeling. Mol. Neurodegener. 7, 14 (2012).
Baker-Nigh, A. T. et al. Human central nervous system (CNS) apoE isoforms are increased by age, differentially altered by amyloidosis, and relative amounts reversed in the CNS compared with plasma. J. Biol. Chem. 291, 27204–27218 (2016).
Crisp, M. J. et al. In vivo kinetic approach reveals slow SOD1 turnover in the CNS. J. Clin. Invest. 125, 2772–2780 (2015).
Self, W. K. et al. Protein production is an early biomarker for RNA-targeted therapies. Ann. Clin. Transl Neurol. 5, 1492–1504 (2018).
Buee, L. et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 33, 95–130 (2000).
Sato, C. et al. Tau kinetics in neurons and the human central nervous system. Neuron 97, 1284–1298 (2018).
Karch, C. M., Jeng, A. T. & Goate, A. M. Extracellular tau levels are influenced by variability in tau that is associated with tauopathies. J. Biol. Chem. 287, 42751–42762 (2012).
Pooler, A. M. et al. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 (2013).
Blennow, K. et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 (2010).
Gonzalez-Marrero, I. et al. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front. Cell Neurosci. 9, 17 (2015).
Zlokovic, B. V. Cerebrovascular effects of apolipoprotein e implications for Alzheimer disease. JAMA Neurol. 70, 440–444 (2013).
Selkoe, D. J. Alzheimer’s disease is a synaptic failure. Science 298, 789–791 (2002).
Hellwig, K. et al. Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimers Res. Ther. 7, 74 (2015).
Kester, M. I. et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 72, 1275–1280 (2015).
Kvartsberg, H. et al. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimers Res. Ther. 7, 40 (2015).
Rosengren, L. E. et al. Neurofilament protein levels in CSF are increased in dementia. Neurology 52, 1090–1093 (1999).
Kuhle, J. et al. Blood neurofilament light chain levels are elevated in multiple sclerosis and correlate with disease activity [abstract 249]. Mult. Scler. 22, S828 (2016).
Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 (2006).
Millecamps, S. et al. Conditional NF-L transgene expression in mice for in vivo analysis or turnover and transport rate of neurofilaments. J. Neurosci. 27, 4947–4956 (2007).
Guerreiro, R. & Hardy, J. TREM2 and neurodegenerative disease. N. Engl. J. Med. 369, 1569–1570 (2013).
Griciuc, A. et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78, 631–643 (2013).
Suarez-Calvet, M. et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 8, 466–476 (2016).
Schutzer, S. E. et al. Establishing the proteome of normal human cerebrospinal fluid. PLOS ONE 5, e10980 (2010).
Lehmann, S. et al. Stable isotope labeling by amino acid in vivo (SILAV): a new method to explore protein metabolism. Rapid. Commun. Mass Spectrom. 29, 1917–1925 (2015).
Acknowledgements
R.W.P. is supported by a UK National Institute for Health Research (NIHR) academic clinical lectureship. H.Z. is a Wallenberg Academy Fellow and is supported by grants from the Swedish Research Council, the European Research Council, the Olav Thon Foundation and the UK Dementia Research Institute at University College London (UCL). A.G., C.H. and S.L.’s stable isotope labelling kinetics (SILK) work is supported by the French 2010 National Programme Hospitalier de Recherche Clinique (PHRC) ‘ProMara’, Direction de L’Hospitalisation et de l’Organisation des Soins (DHOS). S.W. is supported by an Alzheimer’s Research UK Senior Research Fellowship (ARUK-SRF2016B-2). B.P.L. is supported by a grant from the US National Institute on Aging (K76 AG054863). T.M.’s SILK studies are supported by US NIH grant R01-NS098716-01. C.M.K. is supported by NIH grant K01AG046374. N.C.F. acknowledges support from the NIHR UCL Hospitals Biomedical Research Centre, the Leonard Wolfson Experimental Neurology Centre and the UK Dementia Research Institute at UCL. R.J.B.’s SILK work was supported by the BrightFocus Foundation (grant A2014384S), Tau SILK Consortium (AbbVie, Biogen and Eli Lilly), NIH grant R01-NS095773, MetLife Foundation, Alzheimer’s Association Zenith Award Grant (institution grant #3856-80569), NIH (R01NS065667) and the Cure Alzheimer’s Fund.
Author information
Authors and Affiliations
Contributions
R.W.P., A.G., B.P.L., N.R.B., C.A.L., S.L., C.S., B.W.P., T.W., K.Y., J.D.R., N.C.W., J.M.S., T.M., D.L.E., H.Z., N.C.F. and R.J.B. researched data for the article. R.W.P., A.G., B.P.L., N.R.B., C.S., C.A.L., C.H., S.L., B.W.P., T.W., K.Y., N.C.W., D.L.E. and H.Z. wrote the first draft of the manuscript. R.W.P., J.D.R., J.M.S., S.W., N.C.F. and R.J.B. reviewed and critically edited the manuscript. All authors contributed substantially to discussions of the article content and to the review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.Z. declares that he has served on scientific advisory boards for Roche Diagnostics, Samumed, CogRx and Wave and is one of the founders of Brain Biomarker Solutions in Gothenburg, which is funded by GU Ventures (a Swedish government-owned company managed by the University of Gothenburg); these activities are all unrelated to this article. R.J.B. declares that he, along with Washington University, has an equity ownership interest in C2N Diagnostics (a mass-spectrometry-based biotechnology company that holds patents on the stable isotope labelling kinetics (SILK) technique in the United States and other countries) and receives royalties related to SILK and blood plasma assay technologies licensed by Washington University to C2N Diagnostics. R.J.B. declares that he receives income from C2N Diagnostics for serving on its scientific advisory board. B.W.P. declares that he receives consultancy fees from C2N Diagnostics. T.M. and R.J.B. have licensed superoxide dismutase 1 SILK to C2N Diagnostics. N.C.W. holds a patent for SILK studies utilizing nanoscale secondary ion mass spectroscopy. K.Y. and T.W. declare that they are employed by C2N Diagnostics. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
University College London (UCL) Dementia Research Centre: https://www.ucl.ac.uk/drc
Washington University School of Medicine in St Louis (WUSTL) Department of Neurology, Bateman Laboratory: https://neuro.wustl.edu/labs/bateman_r/Ongoing-Studies
Supplementary information
41582_2019_222_MOESM1_ESM.mov
Supplementary Movie 1 One-compartment model of isotope labeling with varying synthesis rates. Each panel in the top row shows ~10,000 particles in a volume. Because particle formation and removal are determined using a random number generator, the total number of particles might diverge slightly from 10,000. New particles have a given probability of forming at each time increment, and existing particles have a given probability of being removed at each time increment. The synthesis rate increases with increasing probability of particle formation. The degradation (or clearance) rate increases with increasing probability of particle removal. At time 0, all 10,000 particles are nonlabelled (red). The bottom row illustrates fractional labeling — the number of unlabeled versus labeled (blue) particles. Each new particle is assumed to have a chance of being labeled or nonlabeled that is dictated by the mole fraction of labeled precursor (green curve). This assumption is justified by the fact that incorporation of labeled or nonlabeled amino acids relies on competition for binding sites on transfer RNA. Faster synthesis rates result in an increased rate of production of new particles. However, the overall system is assumed to be at a steady state. If degradation rates are equal, the ‘fast synthesis’ system simply has more total particles than the ‘slow synthesis’ system. Because the ‘slow synthesis’ system starts with fewer particles, the slower synthesis of labeled particles is negated when calculating or measuring the mole fraction of labeled particles. Thus, the kinetic curves for the fast and slow synthesis systems overlap (except for stochastic variations due to the relatively small number of particles in the model).
41582_2019_222_MOESM2_ESM.mov
Supplementary Movie 2 One-compartment model of isotope labeling with varying degradation rates. Fewer particles also result from fast degradation (right panel) if synthesis rates are the same. Because new particles are synthesized at the same rate, the mole fraction of labeled particles increases at a greater rate when fast degradation is present. The fast-degradation system simply has fewer unlabeled particles. Initially, the number of labeled particles is nearly identical in the fast-degradation and slow-degradation situations, but the effect of rapid degradation becomes evident over time. The net result is that the fast-degradation system peaks earlier and higher and declines faster. Stable isotobe labeling kinetics (SILK) directly measure degradation rates, but indirectly measure synthesis rates (because synthesis rates can only be determined if the total concentration of particles is known). At steady state, the total concentration of particles is equal to the degradation (turnover) rate constant divided by the synthesis rate constant.
41582_2019_222_MOESM3_ESM.mov
Supplementary Movie 3 Three-compartment model of isotope labeling. In this model, synthesis and degradation rate constants are identical for all three compartments (APP, C99 and Aβ42). The probability of new APP particles being labeled or nonlabeled is dictated by the mole fraction of labeled precursor (blue curve). APP particles move at a certain rate into the C99 compartment, which simultaneously represents both degradation of APP and synthesis of C99. Similarly, C99 is converted to Aβ42, which is degraded at a certain rate and disappears from the system. The kinetic plot of mole fraction labeled peptides shows that sequential synthesis results in a delay in the onset of production of labeled C99 and labeled Aβ42. Although APP concentration shows a monoexponential rise to plateau, C99 and Aβ42 curves have sigmoidal shapes during the labeling phase. After administration of the labeled precursor halts, the mole fraction of labeled APP immediately decreases and this curve shows a monoexponential decay. After some delay, the C99 and Aβ42 curves also show monoexponential decay. This very simple model captures the typical shape of in vivo stable isotope labeling kinetics (SILK) amyloid-β (Aβ) curves. Most Aβ-SILK curves are consistent with a single rate-limiting compartment serially connected to about five other compartments. Aβ42, amyloid-β protein 42, APP, amyloid-β precursor protein; C99, β-secretase C-terminal fragment.
Rights and permissions
About this article
Cite this article
Paterson, R.W., Gabelle, A., Lucey, B.P. et al. SILK studies — capturing the turnover of proteins linked to neurodegenerative diseases. Nat Rev Neurol 15, 419–427 (2019). https://doi.org/10.1038/s41582-019-0222-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-019-0222-0
This article is cited by
-
Stable isotope labeling and ultra-high-resolution NanoSIMS imaging reveal alpha-synuclein-induced changes in neuronal metabolism in vivo
Acta Neuropathologica Communications (2023)
-
The metabolism of human soluble amyloid precursor protein isoforms is quantifiable by a stable isotope labeling-tandem mass spectrometry method
Scientific Reports (2022)
-
Early-stage Alzheimer disease: getting trial-ready
Nature Reviews Neurology (2022)
-
Relevance of biomarkers across different neurodegenerative diseases
Alzheimer's Research & Therapy (2020)