Abstract
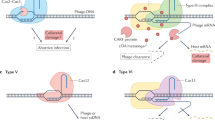
The principal function of CRISPR–Cas systems in archaea and bacteria is defence against mobile genetic elements (MGEs), including viruses, plasmids and transposons. However, the relationships between CRISPR–Cas and MGEs are far more complex. Several classes of MGE contributed to the origin and evolution of CRISPR–Cas, and, conversely, CRISPR–Cas systems and their components were recruited by various MGEs for functions that remain largely uncharacterized. In this Analysis article, we investigate and substantially expand the range of CRISPR–Cas components carried by MGEs. Three groups of Tn7-like transposable elements encode ‘minimal’ type I CRISPR–Cas derivatives capable of target recognition but not cleavage, and another group encodes an inactivated type V variant. These partially inactivated CRISPR–Cas variants might mediate guide RNA-dependent integration of the respective transposons. Numerous plasmids and some prophages encode type IV systems, with similar predicted properties, that appear to contribute to competition among plasmids and between plasmids and viruses. Many prokaryotic viruses also carry CRISPR mini-arrays, some of which recognize other viruses and are implicated in inter-virus conflicts, and solitary repeat units, which could inhibit host CRISPR–Cas systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Makarova, K. S. et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Mohanraju, P. et al. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science 353, aad5147 (2016).
Barrangou, R. & Horvath, P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2, 17092 (2017).
Jackson, S. A. et al. CRISPR-Cas: adapting to change. Science 356, eaal5056 (2017).
Koonin, E. V., Makarova, K. S. & Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 37, 67–78 (2017). This article is the latest published overview of the CRISPR–Cas diversity, with an emphasis on Class 2 systems discovered through dedicated search efforts.
Garcia-Martinez, J., Maldonado, R. D., Guzman, N. M. & Mojica, F. J. M. The CRISPR conundrum: evolve and maybe die, or survive and risk stagnation. Microb. Cell 5, 262–268 (2018).
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Classification and nomenclature of CRISPR-Cas systems: where from here? CRISPR J. https://doi.org/10.1089/crispr.2018.0033 (2018).
Faure, G., Makarova, K. S. & Koonin, E. V. CRISPR-Cas: complex functional networks and multiple roles beyond adaptive immunity. J. Mol. Biol. 431, 3–20 (2018).
Westra, E. R., Buckling, A. & Fineran, P. C. CRISPR-Cas systems: beyond adaptive immunity. Nat. Rev. Microbiol. 12, 317–326 (2014).
Shmakov, S. A., Makarova, K. S., Wolf, Y. I., Severinov, K. V. & Koonin, E. V. Systematic prediction of genes functionally linked to CRISPR-Cas systems by gene neighborhood analysis. Proc. Natl Acad. Sci. USA 115, E5307–E5316 (2018). This article presents systematic prediction and analysis of genes associated with various subsets of CRISPR–Cas systems. The results suggest substantial functional diversification of CRISPR–Cas, in particular, coupling with signal transduction, especially in type III systems.
Shah, S. A. et al. Comprehensive search for accessory proteins encoded with archaeal and bacterial type III CRISPR-cas gene cassettes reveals 39 new cas gene families. RNA Biol. https://doi.org/10.1080/15476286.2018.1483685 (2018). This paper complements Shmakov et al. (2018) by providing systematic analysis of predicted accessory proteins associated with type III CRISPR–Cas systems.
Koonin, E. V. & Makarova, K. S. Mobile genetic elements and evolution of CRISPR-Cas systems: all the way there and back. Genome Biol. Evol 9, 2812–2825 (2017).
Krupovic, M., Beguin, P. & Koonin, E. V. Casposons: mobile genetic elements that gave rise to the CRISPR-Cas adaptation machinery. Curr. Opin. Microbiol. 38, 36–43 (2017).
Krupovic, M., Makarova, K. S., Forterre, P., Prangishvili, D. & Koonin, E. V. Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity. BMC Biol. 12, 36 (2014).
Kazazian, H. H. Jr. Mobile elements: drivers of genome evolution. Science 303, 1626–1632 (2004).
Gueguen, E., Rousseau, P., Duval-Valentin, G. & Chandler, M. The transpososome: control of transposition at the level of catalysis. Trends Microbiol. 13, 543–549 (2005).
Peters, J. E. & Craig, N. L. Tn7: smarter than we thought. Nat. Rev. Mol. Cell Biol. 2, 806–814 (2001).
Fricker, A. D. & Peters, J. E. Vulnerabilities on the lagging-strand template: opportunities for mobile elements. Annu. Rev. Genet. 48, 167–186 (2014).
Nunez, J. K., Lee, A. S., Engelman, A. & Doudna, J. A. Integrase-mediated spacer acquisition during CRISPR-Cas adaptive immunity. Nature 519, 193–198 (2015).
Hudaiberdiev, S. et al. Phylogenomics of Cas4 family nucleases. BMC Evol. Biol. 17, 232 (2017).
Shmakov, S. et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol. Cell 60, 385–397 (2015).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017). This article presents a definitive description of the dedicated efforts on the discovery of diverse Class 2 CRISPR–Cas systems. The key finding is the identification of multiple variants assigned to subtype V-U that appear to have independently evolved from different groups of TnpB nucleases and are likely to be evolutionary intermediates on the path from TnpB to bona fide Class 2 CRISPR effectors.
Peters, J. E., Makarova, K. S., Shmakov, S. & Koonin, E. V. Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc. Natl Acad. Sci. USA 114, E7358–E7366 (2017). This article is the first description of derived CRISPR–Cas systems carried by Tn7-like transposons. A hypothetical mechanism for crRNA-guided transposition is proposed.
McDonald, N. D., Regmi, A., Morreale, D. P., Borowski, J. D. & Fidelma Boyd, E. CRISPR-Cas systems are present predominantly on mobile genetic elements in Vibrio species. BMC Genomics 20, 105 (2019).
Ozcan, A. et al. Type IV CRISPR RNA processing and effector complex formation in Aromatoleum aromaticum. Nat. Microbiol. 4, 89–96 (2019). This article presents the most thorough available characterization of the structure and biochemical activities of type IV CRISPR–Cas systems. The similarity of the effector complex structure to those of type I is demonstrated, suggesting that type IV is an extremely derived form of type I.
Maier, L. K., Dyall-Smith, M. & Marchfelder, A. The adaptive immune system of Haloferax volcanii. Life (Basel) 5, 521–537 (2015).
Seed, K. D., Lazinski, D. W., Calderwood, S. B. & Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489–491 (2013).
Naser, I. B. et al. Analysis of the CRISPR-Cas system in bacteriophages active on epidemic strains of Vibrio cholerae in Bangladesh. Sci. Rep. 7, 14880 (2017).
Roberts, A. P. & Mullany, P. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 35, 856–871 (2011).
Parks, A. R. et al. Transposition into replicating DNA occurs through interaction with the processivity factor. Cell 138, 685–695 (2009).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018). This article is an experimental validation of the interference activity of three small type V effector proteins that are closely related to TnpB and some of the subtype V-U variants described in Shmakov et al. ( Nat. Rev. Microbiol. , 2017). Preferential activity against single-stranded DNA, as opposed to double-stranded DNA, as is the case for Cas12, is demonstrated. The corresponding CRISPR–Cas type systems are now classified as subtype V-F.
Yan, W. X. et al. Functionally diverse type V CRISPR-Cas systems. Science 363, 88–91 (2019). This work complements Harrington et al. (2018) by demonstrating the activity of a distinct V-U variant (reclassified subtype V-G) that unexpectedly shows strong preference for single-stranded RNA substrates.
He, S. et al. The IS200/IS605 family and “peel and paste” single-strand transposition mechanism. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.MDNA3-0039-2014 (2015).
Chen, J. S. et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018).
Choi, K. Y., Spencer, J. M. & Craig, N. L. The Tn7 transposition regulator TnsC interacts with the transposase subunit TnsB and target selector TnsD. Proc. Natl Acad. Sci. USA 111, E2858–E2865 (2014).
Peters, J. E. Tn7. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.MDNA3-0010-2014 (2014).
Koonin, E. V. & Krupovic, M. Evolution of adaptive immunity from transposable elements combined with innate immune systems. Nat. Rev. Genet. 16, 184–192 (2015).
Nowacki, M., Shetty, K. & Landweber, L. F. RNA-mediated epigenetic programming of genome rearrangements. Annu. Rev. Genomics Hum. Genet. 12, 367–389 (2011).
Newire, E., Aydin, A., Juma, S., Enne, V. & Roberts, A. P. Identification of a type IV CRISPR-Cas system located exclusively on IncHI1B/ IncFIB plasmids in Enterobacteriaceae. Preprint at bioRxiv https://doi.org/10.1101/536375 (2019)
Carroll, K. S. et al. A conserved mechanism for sulfonucleotide reduction. PLOS Biol. 3, e250 (2005).
You, D., Wang, L., Yao, F., Zhou, X. & Deng, Z. A novel DNA modification by sulfur: DndA is a NifS-like cysteine desulfurase capable of assembling DndC as an iron-sulfur cluster protein in Streptomyces lividans. Biochemistry 46, 6126–6133 (2007).
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Comparative genomics of defense systems in archaea and bacteria. Nucleic Acids Res. 41, 4360–4377 (2013).
Simon, N. C., Aktories, K. & Barbieri, J. T. Novel bacterial ADP-ribosylating toxins: structure and function. Nat. Rev. Microbiol. 12, 599–611 (2014).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Shabbir, M. A. et al. Bacteria versus bacteriophages: parallel evolution of immune arsenals. Front. Microbiol. 7, 1292 (2016).
Villion, M. & Moineau, S. The double-edged sword of CRISPR-Cas systems. Cell Res. 23, 15–17 (2013).
Angermeyer, A., Das, M. M., Singh, D. V. & Seed, K. D. Analysis of 19 highly conserved Vibrio cholerae bacteriophages isolated from environmental and patient sources over a twelve-year period. Viruses 10, E299 (2018).
Al-Shayeb, B. et al. Clades of huge phage from across Earth’s ecosystems. Preprint at bioRxiv https://doi.org/10.1101/572362 (2019).
Hooton, S. P., Brathwaite, K. J. & Connerton, I. F. The bacteriophage carrier state of Campylobacter jejuni features changes in host non-coding RNAs and the acquisition of new host-derived CRISPR spacer sequences. Front. Microbiol. 7, 355 (2016).
Hooton, S. P. & Connerton, I. F. Campylobacter jejuni acquire new host-derived CRISPR spacers when in association with bacteriophages harboring a CRISPR-like Cas4 protein. Front. Microbiol. 5, 744 (2014).
He, F. et al. Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity. Nat. Microbiol. 3, 461–469 (2018).
Koonin, E. V. & Makarova, K. S. Anti-CRISPRs on the march. Science 362, 156–157 (2018).
Sebaihia, M. et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38, 779–786 (2006).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Garcia-Heredia, I. et al. Reconstructing viral genomes from the environment using fosmid clones: the case of haloviruses. PLOS ONE 7, e33802 (2012).
Faure, G. et al. Comparative genomics and evolution of trans-activating RNAs in class 2 CRISPR-Cas systems. RNA Biol. https://doi.org/10.1080/15476286.2018.1493331 (2018).
Shmakov, S. A. et al. The CRISPR spacer space is dominated by sequences from species-specific mobilomes. mBio 8, e01397-17 (2017).
Anderson, E. M. et al. Systematic analysis of CRISPR-Cas9 mismatch tolerance reveals low levels of off-target activity. J. Biotechnol. 211, 56–65 (2015).
Zheng, T. et al. Profiling single-guide RNA specificity reveals a mismatch sensitive core sequence. Sci. Rep. 7, 40638 (2017).
Kleinstiver, B. P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Horvath, P. et al. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 190, 1401–1412 (2008).
Leenay, R. T. et al. Identifying and visualizing functional PAM diversity across CRISPR-Cas systems. Mol. Cell 62, 137–147 (2016).
Zhang, Y. et al. Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. Mol. Cell 50, 488–503 (2013).
Amitai, G. & Sorek, R. CRISPR-Cas adaptation: insights into the mechanism of action. Nat. Rev. Microbiol. 14, 67–76 (2016).
Pawluk, A. et al. Naturally occurring off-switches for CRISPR-Cas9. Cell 167, 1829–1838 (2016).
Pawluk, A., Davidson, A. R. & Maxwell, K. L. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2018).
Maxwell, K. L. The anti-CRISPR story: a battle for survival. Mol. Cell 68, 8–14 (2017).
Varble, A., Meaden, S., Barrangou, R., Westra, E. R. & Marraffini, L. A. Recombination between phages and CRISPR-cas loci facilitates horizontal gene transfer in staphylococci. Nat. Microbiol. https://doi.org/10.1038/s41564-019-0400-2 (2019).
Koonin, E. V. & Krupovic, M. A movable defense. TheScientist https://www.the-scientist.com/features/a-movable-defense-36135 (2015).
Arndt, D. et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44, W16–W21 (2016).
Acknowledgements
G.F., S.A.S., K.S.M. and E.V.K. are supported by funds from the Intramural Research Program of the National Institutes of Health of the USA. S.A.S. was additionally supported by the Russian Foundation for Basic Research (research project 18-34-00012) and a systems biology fellowship from Philip Morris Sales and Marketing. J.E.P. was supported by the US Department of Agriculture National Institute of Food and Agriculture Hatch Project NYC-189438. D.R.C., W.X.Y. and D.A.S. are supported by Arbor Biotechnologies.
Reviewer information
Nature Reviews Microbiology thanks U. Gophna, and other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
G.F., S.A.S., W.X.Y., D.R.C., D.A.S., J.E.P., K.S.M. and E.V.K. researched the data for the article. G.F., W.X.Y., D.R.C., D.A.S., J.E.P., K.S.M. and E.V.K. substantially contributed to the discussion of the content. E.V.K. wrote the article. G.F., W.X.Y., D.R.C., D.A.S., J.E.P., K.S.M. and E.V.K. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.R.C., W.X.Y. and D.A.S. are shareholders of Arbor Biotechnologies. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- CRISPR spacers
-
Unique sequences of 20–60 nucleotides inserted between the repeats in the CRISPR array and employed, as part of the CRISPR RNA, for targeting DNA molecules containing a homologous protospacer.
- CRISPR array
-
A series of direct repeats in bacterial and archaeal genomes interspersed with spacers that are acquired primarily from mobile genetic element DNA.
- crRNAs
-
Short RNAs, produced by processing of the primary transcript of a CRISPR array, that consists of a spacer and portions of the flanking repeats and functions as a guide to target DNA or RNA molecules containing cognate protospacers.
- Mini-arrays
-
Minimal forms of a CRISPR array that consists of a proximal repeat, a spacer and a distal repeat, or, more commonly, a partial repeat; so far identified in virus and provirus genomes.
- Solitary repeat units
-
(SRUs). Short sequences, so far identified in virus and provirus genomes, that are (nearly) identical to a repeat from a CRISPR array.
- CRISPR adaptation module
-
A group of cas genes dedicated to the selection and insertion of new spacers into CRISPR arrays.
- TnpB
-
A nuclease containing a RuvC-like domain that is encoded by numerous transposons (insertion sequences) although not required for transposition.
- IS605-like transposons
-
A family of bacterial and archaeal insertion sequence elements that encode a distinct transposase (TnpA) and often a second nuclease (TnpB) that is also found in numerous non-autonomous IS605-like transposons lacking TnpA.
- Tn7-like transposons
-
A derivative of Tn7 family transposons lacking some accessory genes involved in transposition and in some cases carrying derived CRISPR–Cas systems lacking the interference capacity.
- CRISPR effector module
-
A suite of Cas proteins (Class 1 CRISPR–Cas systems) or a single large protein (Class 2 CRISPR–Cas systems) that are responsible for maturation of the CRISPR RNA and interference.
- R-loops
-
Three-stranded structures that consist of a DNA–RNA hybrid and the displaced single-stranded DNA, formed during transcription and other processes including target recognition by CRISPR–Cas effector complexes (proteins).
- Protospacer
-
A piece of DNA, typically from a mobile genetic element genome, that is inserted into a CRISPR array by the CRISPR adaptation complex, to become a spacer.
- CysH enzymes
-
Enzymes of the adenosine 5′-phosphosulfate reductase family that reduce activated sulfate to sulfite; associated with many type IV CRISPR–Cas systems.
- Exaptation
-
Co-option (recruitment) of a biological entity, such as a protein or DNA sequence, for a role that is distinct from its original function.
- Anti-CRISPR proteins
-
Diverse proteins encoded by many bacterial and archaeal viruses that inhibit the host CRISPR–Cas systems, typically by binding and inactivating the effector complex (protein).
- Transactivating RNAs
-
(tracrRNAs). RNA molecules encoded by all known type II CRISPR–Cas systems and some type V systems that consist of a sequence partially complementary to the corresponding repeat and a unique portion; co-folding of the tracrRNA with the pre-crRNA is essential for crRNA maturation and interference by the respective CRISPR–Cas systems.
- Protospacer adjacent motif
-
(PAM). A short, two-to-three-nucleotide motif, the presence of which next to the protospacer sequence is essential for both adaptation and interference by most of the CRISPR–Cas systems; different PAM sequences are required by different CRISPR-Cas types and subtypes.
Rights and permissions
About this article
Cite this article
Faure, G., Shmakov, S.A., Yan, W.X. et al. CRISPR–Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol 17, 513–525 (2019). https://doi.org/10.1038/s41579-019-0204-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0204-7
This article is cited by
-
CRISPR technologies for genome, epigenome and transcriptome editing
Nature Reviews Molecular Cell Biology (2024)
-
Inhibitors of bacterial immune systems: discovery, mechanisms and applications
Nature Reviews Genetics (2024)
-
Precise genome-editing in human diseases: mechanisms, strategies and applications
Signal Transduction and Targeted Therapy (2024)
-
It takes two to tango with CRISPR: a history and overview of augmenting the technology for genetic engineering
Proceedings of the Indian National Science Academy (2024)
-
Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome
Nature Biotechnology (2023)