Abstract
Cyanobacteria can form dense and sometimes toxic blooms in freshwater and marine environments, which threaten ecosystem functioning and degrade water quality for recreation, drinking water, fisheries and human health. Here, we review evidence indicating that cyanobacterial blooms are increasing in frequency, magnitude and duration globally. We highlight species traits and environmental conditions that enable cyanobacteria to thrive and explain why eutrophication and climate change catalyse the global expansion of cyanobacterial blooms. Finally, we discuss management strategies, including nutrient load reductions, changes in hydrodynamics and chemical and biological controls, that can help to prevent or mitigate the proliferation of cyanobacterial blooms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

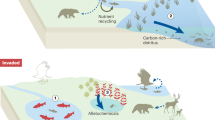
Images in parts b and c courtesy of the European Space Agency, © ESA 2011, CC-BY-SA-3.0 IGO. Image in part d courtesy of L. Krienitz, Leibniz Institute of Freshwater Ecology and Inland Fisheries, Germany. Image in part f courtesy of S. Flury, EAWAG, Switzerland.

Image in part a courtesy of W. van Egmond, Netherlands. Images in parts b–d courtesy of A. Ballot, Norwegian Institute for Water Research (NIVA), Norway. Image in part e courtesy of M. Stomp, University of Amsterdam, Netherlands.


Part a adapted from ref.143, Macmillan Publishers Limited.

Similar content being viewed by others
References
Planavsky, N. J. et al. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 7, 283–286 (2014).
Nutman, A. P., Bennett, V. C., Friend, C. R. L., Van Kranendonk, M. J. & Chivas, A. R. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537, 535–538 (2016).
Schirrmeister, B. E., Gugger, M. & Donoghue, P. C. Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58, 769–785 (2015).
Stomp, M. et al. Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecol. Lett. 10, 290–298 (2007).
Six, C. et al. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: a comparative genomics study. Genome Biol. 8, R259 (2007).
Chorus, I. & Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management (E & FN Spon, London, 1999). This book is a landmark publication that provides an excellent overview of the problems caused by toxic cyanobacteria and puts toxic cyanobacteria on the agenda of water management.
Huisman, J., Matthijs, H. C. P. & Visser, P. M. Harmful Cyanobacteria. (Springer, Berlin, 2005).
Paerl, H. W. & Otten, T. G. Harmful cyanobacterial blooms: causes, consequences and controls. Microb. Ecol. 65, 995–1010 (2013).
Scheffer, M., Hosper, S. H., Meijer, M. L., Moss, B. & Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 8, 275–279 (1993).
Rabalais, N. N. et al. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7, 585–619 (2010).
Izaguirre, G. & Taylor, W. D. A guide to geosmin- and MIB-producing cyanobacteria in the United States. Water Sci. Technol. 49, 19–24 (2004).
Jüttner, F. & Watson, S. B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 73, 4395–4406 (2007).
Carmichael, W. W. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. 7, 1393–1407 (2001).
Metcalf, J. S. & Codd, G. A. in Ecology of Cyanobacteria II: Their Diversity in Space and Time (ed. Whitton, B. A.) 651–675. (Springer, Berlin, 2012).
Merel, S. et al. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 59, 303–327 (2013).
Paerl, H. W. & Huisman, J. Blooms like it hot. Science 320, 57–58 (2008). This concise perspective is one of the first to point out that global warming will favour cyanobacterial blooms.
Jöhnk, K. D. et al. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 14, 495–512 (2008). This study couples a hydrodynamic model and phytoplankton competition model to investigate how an extreme summer heat wave favours surface blooms of harmful cyanobacteria.
Paerl, H. W. & Huisman, J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 1, 27–37 (2009).
Wagner, C. & Adrian, R. Cyanobacteria dominance: quantifying the effects of climate change. Limnol. Oceanogr. 54, 2460–2468 (2009).
Elliott, J. A. The seasonal sensitivity of cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Glob. Chang. Biol. 16, 864–876 (2010).
O’Neil, J. M., Davis, T. W., Burford, M. A. & Gobler, C. J. The rise of harmful cyanobacteria blooms: potential role of eutrophication and climate change. Harmful Algae 14, 313–334 (2012).
Kosten, S. et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 18, 118–126 (2012). This study compares cyanobacterial abundance in 143 lakes along a latitudinal transect from the subarctic to the tropics and shows that the per cent cyanobacteria increases steeply with temperature.
Beaulieu, M., Pick, F. & Gregory-Eaves, I. Nutrients and water temperature are significant predictors of cyanobacterial biomass in a 1147 lakes data set. Limnol. Oceanogr. 58, 1736–1746 (2013).
Michalak, A. M. et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl Acad. Sci. USA 110, 6448–6452 (2013). This study reports how a wet spring causing high nutrient run-off from agriculture, followed by a long, warm summer, led to one of the largest cyanobacterial blooms in the history of Lake Erie.
Verspagen, J. M. H. et al. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLOS ONE 9, e104325 (2014).
Taranu, Z. E. et al. Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecol. Lett. 18, 375–384 (2015). This study analyses more than 100 sedimentary records and shows that cyanobacterial dominance has increased over the past 200 years in temperate and subarctic lakes across the northern hemisphere.
Visser, P. M. et al. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 54, 145–159 (2016).
Chapra, S. C. et al. Climate change impacts on harmful algal blooms in US freshwaters: a screening-level assessment. Environ. Sci. Technol. 51, 8933–8943 (2017).
Przytulska, A., Bartosiewicz, M. & Vincent, W. F. Increased risk of cyanobacterial blooms in northern high-latitude lakes through climate warming and phosphorus enrichment. Freshwater Biol. 62, 1986–1996 (2017).
Shi, K. et al. Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: responses to nutrient enrichment and meteorological factors. Sci. Rep. 7, 40326 (2017).
Ullah, H., Nagelkerken, I., Goldenberg, S. U. & Fordham, D. A. Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PLOS Biol. 16, e2003446 (2018). This study reports on field experiments demonstrating that warming and CO 2 enrichment of a marine ecosystem boost the growth of benthic cyanobacteria, which in turn reduces energy flow to higher trophic levels in the food web.
Qin, B. et al. A drinking water crisis in Lake Taihu, China: linkage to climatic variability and lake management. Environ. Manag. 45, 105–112 (2010).
Guo, L. Doing battle with the green monster of Taihu Lake. Science 317, 1166–1166 (2007).
Duan, H. et al. Two-decade reconstruction of algal blooms in China’s Lake Taihu. Environ. Sci. Technol. 43, 3522–3528 (2009).
Makarewicz, J. C. Phytoplankton biomass and species composition in Lake Erie, 1970 to 1987. J. Great Lakes Res. 19, 258–274 (1993).
Nicholls, K. H. & Hopkins, G. J. Recent changes in Lake Erie (north shore) phytoplankton: cumulative impacts of phosphorus loading reductions and zebra mussel introduction. J. Great Lakes Res. 19, 637–647 (1993).
Stumpf, R. P., Wynne, T. T., Baker, D. B. & Fahnenstiel, G. L. Interannual variability of cyanobacterial blooms in Lake Erie. PLOS ONE 7, e42444 (2012).
Rinta-Kanto, J. M. et al. Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environ. Sci. Technol. 39, 4198–4205 (2005).
Bullerjahn, G. S. et al. Global solutions to regional problems: collecting global expertise to address the problem of harmful cyanobacterial blooms, a Lake Erie case study. Harmful Algae 54, 223–238 (2016).
Bianchi, T. S. et al. Cyanobacterial blooms in the Baltic Sea: natural or human-induced? Limnol. Oceanogr. 45, 716–726 (2000).
Finni, T., Kononen, K., Olsonen, R. & Wallström, K. The history of cyanobacterial blooms in the Baltic Sea. AMBIO 30, 172–178 (2001).
Suikkanen, S., Laamanen, M. & Huttunen, M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuarine Coastal Shelf Sci. 71, 580–592 (2007).
Kahru, M. & Elmgren, R. Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences 11, 3619–3633 (2014). This study illustrates how satellite remote sensing contributes to long-term monitoring of cyanobacterial blooms.
Bergman, B., Sandh, G., Lin, S., Larsson, J. & Carpenter, E. J. Trichodesmium: a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 37, 286–302 (2013).
Spatharis, S., Skliris, N., Meziti, A. & Kormas, K. A. First record of a Trichodesmium erythraeum bloom in the Mediterranean Sea. Can. J. Fish. Aquat. Sci. 69, 1444–1455 (2012).
Paul, V. J., Thacker, R. W., Banks, K. & Golubic, S. Benthic cyanobacterial bloom impacts the reefs of South Florida (Broward County, USA). Coral Reefs 24, 693–697 (2005).
Ford, A. K. et al. Reefs under siege: the rise, putative drivers, and consequences of benthic cyanobacterial mats. Front. Mar. Sci. 5, 18 (2018).
De Bakker, D. M. et al. 40 Years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 36, 355–367 (2017).
Gallon, J. R. Reconciling the incompatible: N2 fixation and O2. New Phytol. 122, 571–609 (1992).
Muro-Pastor, A. M. & Hess, W. R. Heterocyst differentiation: from single mutants to global approaches. Trends Microbiol. 20, 548–557 (2012).
Stal, L. J. Is the distribution of nitrogen-fixing cyanobacteria in the oceans related to temperature? Environ. Microbiol. 11, 1632–1645 (2009).
Walsby, A. E. The permeability of heterocysts to the gases nitrogen and oxygen. Proc. R. Soc. B Biol. Sci. 226, 345–366 (1985).
Brauer, V. S. et al. Low temperature delays timing and enhances the cost of nitrogen fixation in the unicellular cyanobacterium Cyanothece. ISME J. 7, 2105–2115 (2013).
Breitbarth, E., Oschlies, A. & La Roche, J. Physiological constraints on the global distribution of Trichodesmium: effects of temperature on diazotrophy. Biogeosciences 4, 53–61 (2007).
Ibelings, B. W. & Maberly, S. C. Photoinhibition and the availability of inorganic carbon restrict photosynthesis by surface blooms of cyanobacteria. Limnol. Oceanogr. 43, 408–419 (1998).
Price, G. D., Badger, M. R., Woodger, F. J. & Long, B. M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59, 1441–1461 (2008). This study provides an excellent review of the CCMs of cyanobacteria by pioneers in this field.
Burnap, R. L., Hagemann, M. & Kaplan, A. Regulation of CO2 concentrating mechanism in cyanobacteria. Life 5, 348–371 (2015).
Sandrini, G., Matthijs, H. C. P., Verspagen, J. M. H., Muyzer, G. & Huisman, J. Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J. 8, 589–600 (2014).
Sandrini, G. et al. Rapid adaptation of harmful cyanobacteria to rising CO2. Proc. Natl Acad. Sci. USA 112, 9315–9320 (2016). This study demonstrates with selection experiments and field data that increasing CO 2 concentrations induce rapid adaptive changes in the CCM of cyanobacterial blooms.
Walsby, A. E. Gas vesicles. Microbiol. Rev. 58, 94–144 (1994). This classic review is a must-read for everyone interested in the gas vesicles of buoyant cyanobacteria.
Pfeifer, F. Distribution, formation and regulation of gas vesicles. Nat. Rev. Microbiol. 10, 705–715 (2012).
Sommaruga, R., Chen, Y. & Liu, Z. Multiple strategies of bloom-forming Microcystis to minimize damage by solar ultraviolet radiation in surface waters. Microb. Ecol. 57, 667–674 (2009).
Walsby, A. E., Hayes, P. K., Boje, R. & Stal, L. J. The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 136, 407–417 (1997).
Huisman, J. et al. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960–2970 (2004).
Reynolds, C. S., Oliver, R. L. & Walsby, A. E. Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. NZ J. Mar. Freshwater Res. 21, 379–390 (1987).
Visser, P. M., Passarge, J. & Mur, L. R. Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia 349, 99–109 (1997).
Kromkamp, J. C. & Mur, L. R. Buoyant density changes in the cyanobacterium Microcystis aeruginosa due to changes in the cellular carbohydrate content. FEMS Microbiol. Lett. 25, 105–109 (1984).
Ibelings, B. W., Mur, L. R. & Walsby, A. E. Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes. J. Plankton Res. 13, 419–436 (1991).
Villareal, T. A. & Carpenter, E. J. Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microb. Ecol. 45, 1–10 (2003).
Walsby, A. E., Schanz, F. & Schmid, M. The Burgundy-blood phenomenon: a model of buoyancy change explains autumnal waterblooms of Planktothrix rubescens in Lake Zurich. New Phytol. 169, 109–122 (2006).
Meriluoto, J., Spoof, L. & Codd, G. A. (eds). Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. (John Wiley & Sons, Inc., Chichester, 2017). This recent handbook includes reviews on cyanobacterial blooms and cyanotoxins, with standard operating procedures for their monitoring and analysis.
Hairston, Jr. N. G. et al. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution 55, 2203–2214 (2001). This study hatches eggs of the water flea Daphnia from 35 years of sediment, demonstrating that Daphnia developed resistance to toxic cyanobacteria after they became dominant in Lake Constance.
Lemaire, V. et al. Genotype × genotype interactions between the toxic cyanobacterium Microcystis and its grazer, the waterflea Daphnia. Evol. Appl. 5, 168–182 (2012). This interesting study illustrates the co-evolutionary arms race between toxic cyanobacteria and their grazers.
Jiang, X., Gao, H., Zhang, L., Liang, H. & Zhu, X. Rapid evolution of tolerance to toxic Microcystis in two cladoceran grazers. Sci. Rep. 6, 25319 (2016).
Rantala, A. et al. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl Acad. Sci. USA 101, 568–573 (2004).
Zilliges, Y. et al. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLOS ONE 6, e17615 (2011).
Kardinaal, W. E. A. et al. Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquat. Microb. Ecol. 48, 1–12 (2007).
Sabart, M. et al. Spatiotemporal variations in microcystin concentrations and in the proportions of microcystin-producing cells in several Microcystis aeruginosa populations. Appl. Environ. Microbiol. 76, 4750–4759 (2010).
Mantzouki, E. et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 10, 156 (2018). This recent study presents the first large inventory of the geographical distribution of cyanotoxins on a continental scale.
Rapala, J., Sivonen, K., Lyra, C. & Niemelä, S. I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 63, 2206–2212 (1997).
Wiedner, C. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 69, 1475–1481 (2003).
Van de Waal, D. B. et al. The ecological stoichiometry of toxins produced by harmful cyanobacteria an experimental test of the carbon–nutrient balance hypothesis. Ecol. Lett. 12, 1326–1335 (2009).
Kardinaal, W. E. A. & Visser, P. M. in Harmful Cyanobacteria (eds Huisman, J., Matthijs, H. C. P. & Visser, P. M.) 41–64. (Springer, Berlin, 2005).
Kurmayer, R., Christiansen, G., Fastner, J. & Börner, T. Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environ. Microbiol. 6, 831–841 (2004).
MacKintosh, C., Beattie, K. A., Klumpp, S., Cohen, P. & Codd, G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 264, 187–192 (1990).
Yoshizawa, S. et al. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 116, 609–614 (1990).
Falconer, I. R., Beresford, A. M. & Runnegar, M. T. Evidence of liver damage by toxin from a bloom of the blue-green alga, Microcystis aeruginosa. Med. J. Aust. 1, 511–514 (1983).
Jochimsen, E. M. et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 338, 873–878 (1998). This study tells the sad story of more than 50 patients who died from acute liver failure after haemodialysis using water contaminated with cyanotoxins.
Chen, L., Chen, J., Zhang, X. & Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 301, 381–399 (2016).
Miller, M. A. et al. Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters. PLOS One 5, e12576 (2010).
Meissner, S., Fastner, J. & Dittmann, E. Microcystin production revisited: conjugate formation makes a major contribution. Environ. Microbiol. 15, 1810–1820 (2013).
Miles, C. O. et al. Conjugation of microcystins with thiols is reversible: base-catalyzed deconjugation for chemical analysis. Chem. Res. Toxicol. 29, 860–870 (2016).
Pearson, L., Mihali, T., Moffitt, M., Kellmann, R. & Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 8, 1650–1680 (2010).
Hawkins, P. R., Runnegar, M. T., Jackson, A. R. & Falconer, I. R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 50, 1292–1295 (1985).
Padisák, J. Cylindrospermopsis raciborskii (Woloszynska) Seenaya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology. Arch. Hydrobiol. Suppl. Algol. 107, 563–593 (1997).
Antunes, J. T., Leão, P. N. & Vasconcelos, V. M. Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 6, 473 (2015).
Wiese, M., D’Agostino, P. M., Mihali, T. K., Moffitt, M. C. & Neilan, B. A. Neurotoxic alkaloids: saxitoxin and its analogs. Mar. Drugs 8, 2185–2211 (2010).
Lobner, D., Piana, P. M. T., Salous, A. K. & Peoples, R. W. Beta-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 25, 360–366 (2007).
Cox, P. A., Banack, S. A. & Murch, S. J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl Acad. Sci. USA 100, 13380–13383 (2003).
Bradley, W. G. et al. Is exposure to cyanobacteria an environmental risk factor for amyotrophic lateral sclerosis and other neurodegenerative diseases? Amyotroph. Lateral Scler. Frontotemporal Degener. 13, 325–333 (2013).
Durai, P., Batool, M. & Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 13, 4217–4230 (2015).
Neilan, B. A., Pearson, L. A., Muenchhoff, J., Moffitt, M. C. & Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 15, 1239–1253 (2013).
Paerl, H. W. Transfer of N2 and CO2 fixation products from Anabaena oscillarioides to associated bacteria during inorganic carbon sufficiency and deficiency. J. Phycol. 20, 600–608 (1984).
Brauer, V. S. et al. Competition and facilitation between the marine nitrogen-fixing cyanobacterium Cyanothece and its associated bacterial community. Front. Microbiol. 5, 795 (2015).
Ploug, H. et al. Carbon, nitrogen and O2 fluxes associated with the cyanobacterium Nodularia spumigena in the Baltic Sea. ISME J. 5, 1549–1558 (2011).
Hmelo, L. R., van Mooy, B. A. S. & Mincer, R. J. Characterization of bacterial epibionts on the cyanobacterium Trichodesmium. Aquat. Microb. Ecol. 67, 1–14 (2012).
Alvarenga, D. O., Fiore, M. F. & Varani, A. M. A metagenomic approach to cyanobacterial genomics. Front. Microbiol. 8, 809 (2017).
Louati, I. et al. Structural diversity of bacterial communities associated with bloom-forming freshwater cyanobacteria differs according to the cyanobacterial genus. PLOS One 10, e0140614 (2015).
Berg, C. et al. Dissection of microbial community functions during a cyanobacterial bloom in the Baltic Sea via metatranscriptomics. Front. Mar. Sci. 5, 55 (2018).
Van Hannen, E. J. et al. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl. Environ. Microbiol. 65, 795–801 (1999).
Shao, K. et al. The responses of the taxa composition of particle-attached bacterial community to the decomposition of Microcystis blooms. Sci. Total Environ. 488–489, 236–242 (2014).
Gerphagnon, M. et al. Microbial players involved in the decline of filamentous and colonial cyanobacterial blooms with a focus on fungal parasitism. Environ. Microbiol. 17, 2573–2587 (2015).
Van Wichelen, J., Vanormelingen, P., Codd, G. A. & Vyverman, W. The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55, 97–111 (2016).
Yoshida, M. Ecological dynamics of the toxic bloom-forming cyanobacterium Microcystis aeruginosa and its cyanophages in freshwater. Appl. Environ. Microbiol. 74, 3269–3273 (2008).
Coloma, S. E. et al. Newly isolated Nodularia phage influences cyanobacterial community dynamics. Environ. Microbiol. 19, 273–286 (2017).
Kagami, M., de Bruin, A., Ibelings, B. W. & Van Donk, E. Parasitic chytrids: their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 578, 113–129 (2007).
Makarova, K. S., Wolf, Y. I., Snir, S. & Koonin, E. V. Defense islands in bacterial and archaeal genomes and prediction of novel defense systems. J. Bacteriol. 193, 6039–6056 (2011).
Kuno, S., Sako, Y. & Yoshida, T. Diversification of CRISPR within coexisting genotypes in a natural population of the bloom-forming cyanobacterium Microcystis aeruginosa. Microbiology 160, 903–916 (2014).
Rohrlack, T., Christiansen, G. & Kurmayer, R. Putative antiparasite defensive system involving ribosomal and nonribosomal oligopeptides in cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 79, 2642–2647 (2013).
Kimura, S., Sako, Y. & Yoshida, T. Rapid gene diversification of Microcystis cyanophages revealed by long-and short-term genetic analysis of the tail sheath gene in a natural pond. Appl. Environ. Microbiol. 79, 2789–2795 (2013).
DeMott, W. R., Gulati, R. D. & Van Donk, E. Daphnia food limitation in three hypereutrophic Dutch lakes: evidence for exclusion of large-bodied species by interfering filaments of cyanobacteria. Limnol. Oceanogr. 46, 2054–2060 (2001).
Gliwicz, Z. M. & Lampert, W. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71, 691–702 (1990).
Müller-Navarra, D. C., Brett, M. T., Liston, A. M. & Goldman, C. R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403, 74–77 (2000).
Martin-Creuzburg, D., von Elert, E. & Hoffmann, K. H. Nutritional constraints at the cyanobacteria-Daphnia magna interface: the role of sterols. Limnol. Oceanogr. 53, 456–468 (2008).
Rohrlack, T., Dittmann, E., Henning, M., Börner, T. & Kohl, J. G. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 65, 737–739 (1999).
Sadler, T. & von Elert, E. Physiological interaction of Daphnia and Microcystis with regard to cyanobacterial secondary metabolites. Aquat. Toxicol. 156, 96–105 (2014).
Burian, A., Kainz, M. J., Schagerl, M. & Yasindi, A. Species-specific separation of lake plankton reveals divergent food assimilation patterns in rotifers. Freshwater Biol. 59, 1257–1265 (2014).
Groendahl, S. & Fink, P. High dietary quality of non-toxic cyanobacteria for a benthic grazer and its implications for the control of cyanobacterial biofilms. BMC Ecol. 17, 20 (2017).
Chislock, M. F., Sarnelle, O., Jernigan, L. M. & Wilson, A. E. Do high concentrations of microcystin prevent Daphnia control of phytoplankton? Water Res. 47, 1961–1970 (2013).
Vollenweider, R. A. Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters, with Particular Reference to Nitrogen and Phosphorus as Factors in Eutrophication (OECD, Paris, 1968).
Schindler, D. W. Eutrophication and recovery in experimental lakes: implications for lake management. Science 184, 897–899 (1974).
Jeppesen, E. et al. Lake responses to reduced nutrient loading: an analysis of contemporary long-term data from 35 case studies. Freshwater Biol. 50, 1747–1771 (2005).
Fastner, J. et al. Combating cyanobacterial proliferation by avoiding or treating inflows with high P load: experiences from eight case studies. Aquat. Ecol. 50, 367–383 (2016). This study presents a very nice overview of eight lakes in which cyanobacterial blooms were successfully controlled through the reduction of phosphorus loads.
Grizzetti, B., Bouraoui, F. & Aloe, A. Changes of nitrogen and phosphorus loads to European seas. Glob. Change Biol. 18, 769–782 (2012).
Glibert, P. M., Maranger, R., Sobota, D. J. & Bouwman, L. The Haber Bosch–harmful algal bloom (HB–HAB) link. Environ. Res. Lett. 9, 105001 (2014).
Donald, D. B., Bogard, M. J., Finlay, K. & Leavitt, P. R. Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnol. Oceanogr. 56, 2161–2175 (2011).
Posch, T., Köster, O., Salcher, M. M. & Pernthaler, J. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat. Clim. Change 2, 809–813 (2012).
Gobler, C. J. et al. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 54, 87–97 (2016).
Shapiro, J. Current beliefs regarding dominance of bluegreens: the case for the importance of CO2 and pH. Verhandlungen Intern. Vereinig. Theoretische Angewandte Limnol. 24, 38–54 (1990).
Low-Décarie, E., Fussmann, G. F. & Bell, G. The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Glob. Change Biol. 17, 2525–2535 (2011).
Ji, X., Verspagen, J. M. H., Stomp, M. & Huisman, J. Competition between cyanobacteria and green algae at low versus elevated CO2: who will win, and why? J. Exp. Bot. 68, 3815–3828 (2017).
Hutchins, D. A., Fu, F. X., Webb, E. A., Walworth, N. & Tagliabue, A. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nat. Geosci. 6, 790–795 (2013).
Thomas, M. K. & Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 763, 357–369 (2016).
Taranu, Z. E., Zurawell, R. W., Pick, F. & Gregory-Eaves, I. Predicting cyanobacterial dynamics in the face of global change: the importance of scale and environmental context. Glob. Change Biol. 18, 3477–3490 (2012).
Rigosi, A., Carey, C. C., Ibelings, B. W. & Brookes, J. D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 59, 99–114 (2014).
Anneville, O., Domaizon, I., Kerimoglu, O., Rimet, F. & Jacquet, S. Blue-green algae in a “Greenhouse Century”? New insights from field data on climate change impacts on cyanobacteria abundance. Ecosystems 18, 441–458 (2015).
Stocker, T. F. et al. Climate change 2013: The physical science basis. Intergovernmental Panel on Climate Change, Working Group I Contribution to the IPCC Fifth Assessment Report (AR5) (Cambridge Univ. Press, New York, 2013).
Reichwaldt, E. S. & Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: between simplistic scenarios and complex dynamics. Water Res. 46, 1372–1393 (2012).
Lehman, P. W. et al. Impacts of the 2014 severe drought on the Microcystis bloom in San Francisco Estuary. Harmful Algae 63, 94–108 (2017).
Søndergaard, M., Jensen, J. P. & Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506, 135–145 (2003).
Ibelings, B. W. et al. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10, 4–16 (2007).
Lürling, M. & Faassen, E. J. Controlling toxic cyanobacteria: effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 46, 1447–1459 (2012).
Copetti, D. et al. Eutrophication management in surface waters using lanthanum modified bentonite: a review. Water Res. 97, 162–174 (2016).
Berg, U., Neumann, T., Donnert, D., Nüesch, R. & Stüben, D. Sediment capping in eutrophic lakes: efficiency of undisturbed calcite barriers to immobilize phosphorus. Appl. Geochem. 19, 1759–1771 (2004).
Paerl, H. W. et al. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54, 213–222 (2016).
Visser, P. M., Ibelings, B. W., Van der Veer, B., Koedood, J. & Mur, L. R. Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Nieuwe Meer, the Netherlands. Freshwater Biol. 36, 435–450 (1996).
Visser, P. M., Ibelings, B. W., Bormans, M. & Huisman, J. Artificial mixing to control cyanobacterial blooms: a review. Aquat. Ecol. 50, 423–441 (2016).
Verspagen, J. M. H. et al. Water management strategies against toxic Microcystis blooms in the Dutch delta. Ecol. Appl. 16, 313–327 (2006).
Mitrovic, S. M., Hardwick, L. & Dorani, F. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J. Plankton Res. 33, 229–241 (2011).
Matthijs, H. C. P., Jančula, D., Visser, P. M. & Maršálek, B. Existing and emerging cyanocidal compounds: new perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 50, 443–460 (2016).
Kenefick, S. L., Hrudey, S. E., Peterson, H. G. & Prepas, E. E. Toxin release from Microcystis aeruginosa after chemical treatment. Water Sci. Technol. 27, 433–440 (1993).
Matthijs, H. C. P. et al. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 46, 1460–1472 (2012). This study is the first demonstration that low concentrations of hydrogen peroxide can effectively eliminate a cyanobacterial bloom from an entire lake, without major direct effects on eukaryotic phytoplankton, zooplankton or macrofauna.
Barrington, D. J., Reichwaldt, E. S. & Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 50, 86–94 (2013).
Drábková, M., Matthijs, H. C. P., Admiraal, W. & Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 45, 363–369 (2007).
Reeders, H. H., Bij de Vaate, A. & Slim, F. J. The filtration rate of Dreissena polymorpha (Bivalvia) in three Dutch lakes with reference to biological water quality management. Freshwater Biol. 22, 133–141 (1989).
Waajen, G. W. A. M., Van Bruggen, N. C. B., Dionisio Pires, L. M., Lengkeek, W. & Lürling, M. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecol. Eng. 90, 141–150 (2016).
Dionisio Pires, L. M., Bontes, B. M., Van Donk, E. & Ibelings, B. W. Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. J. Plankton Res. 27, 331–339 (2005).
Vanderploeg, H. A. et al. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can. J. Fish. Aquat. Sci. 58, 1208–1221 (2001).
White, J. D. & Sarnelle, O. Size-structured vulnerability of the colonial cyanobacterium. Microcystis aeruginosa, to grazing by zebra mussels (Dreissena polymorpha). Freshwater Biol. 59, 514–525 (2014).
Conroy, J. D. et al. Temporal trends in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. J. Great Lakes Res. 31, 89–110 (2005).
Sarnelle, O., White, J. D., Horst, G. P. & Hamilton, S. K. Phosphorus addition reverses the positive effect of zebra mussels (Dreissena polymorpha) on the toxic cyanobacterium, Microcystis aeruginosa. Water Res. 46, 3471–3478 (2012).
With, J. S. & Wright, D. I. Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshwater Biol. 14, 371–383 (1984).
Van De Bund, W. J. & Van Donk, E. Short-term and long-term effects of zooplanktivorous fish removal in a shallow lake: a synthesis of 15 years of data from Lake Zwemlust. Freshwater Biol. 47, 2380–2387 (2002).
Søndergaard, M. et al. Lake restoration: successes, failures and long-term effects. J. Appl. Ecol. 44, 1095–1105 (2007).
Søndergaard, M., Lauridsen, T. L., Johansson, L. S. & Jeppesen, E. Repeated fish removal to restore lakes: case study of Lake Væng, Denmark, two biomanipulations during 30 years of monitoring. Water 9, 43 (2017).
Braun, A. & Pfeiffer, T. Cyanobacterial blooms as the cause of a Pleistocene large mammal assemblage. Paleobiology 28, 139–154 (2002).
Koenigswald, W. V., Braun, A. & Pfeiffer, T. Cyanobacteria and seasonal death: a new taphonomic model for the Eocene Messel lake. Paläontol. Z. 78, 417–424 (2004).
De Boer, E. J. et al. A deadly cocktail: how a drought around 4200 cal. yr BP caused mass mortality events at the infamous ‘dodo swamp’ in Mauritius. Holocene 25, 758–771 (2015).
Fogg, G. E., Stewart, W. D. P., Fay, P. & Walsby, A. E. The Blue-Green Algae (Academic Press, London, 1973).
Kirkby, C. A relation of an inland sea, near Danzick, yielding at a certain season of the year a green substance, which causeth certain death; together with an observation about white amber: communicated by Mr. Kirkby, in a letter written to the publisher from Danzick Decemb. 19, 1671. Phil. Trans. R. Soc. 7, 4069–4070 (1672).
Codd, G. A., Pliński, M., Surosz, W., Hutson, J. & Fallowfield, H. J. Publication in 1672 of animal deaths at the Tuchomskie Lake, northern Poland and a likely role of cyanobacterial blooms. Toxicon 108, 285–286 (2015).
Van Leeuwenhoek, A. Letter of September, 7, 1974, to the Royal Society. Phil. Trans. R. Soc. 9, 178–182 (1674).
Van Egmond, W. The riddle of the ‘green streaks’: in search of the first microorganism which Antoni van Leeuwenhoek described. MicScape Magazine http://www.microscopy-uk.org.uk/mag/artfeb16/wimleeuwenhoek2.html (2016). In this online magazine, the Dutch microscopist Wim van Egmond argues convincingly that Antonie van Leeuwenhoek observed cyanobacterial cells in 1674, 2 years before his often cited ‘discovery of bacteria’.
Francis, G. Poisonous Australia lake. Nature 18, 11–12 (1878).
Codd, G. A., Morton, H. & Baker, P. D. George Francis: a pioneer in the investigation of the quality of South Australia’s drinking water sources (1878–1883). Trans. R. Soc. S. Aust. 139, 164–170 (2015).
Krienitz, L. et al. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 43, 141–148 (2003).
Cox, P. A. et al. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl Acad. Sci. USA 102, 5074–5078 (2005).
Faassen, E. J. Presence of the neurotoxin BMAA in aquatic ecosystems: what do we really know? Toxins 6, 1109–1138 (2014).
Acknowledgements
The authors thank J. van Arkel for help with the drawings and A. Ballot, W. van Egmond, S. Flury, E. Killer, L. Krienitz and M. Stomp for sharing their photographs. H.W.P. was supported by the US National Science Foundation and the Chinese Ministry of Science and Technology. J.M.H.V. was supported by Amsterdam Water Science, which was funded by the Amsterdam Academic Alliance.
Reviewer information
Nature Reviews Microbiology thanks B. Neilan, B. Qin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.H. researched data for the article. J.H., G.A.C., H.W.P., J.M.H.V. and P.M.V. wrote the article. All authors contributed substantially to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Eutrophication
-
The excessive enrichment of ecosystems with dissolved nutrients (for example, nitrate and phosphate), usually through human activity.
- Phytoplankton
-
Microscopically small photosynthetic algae, such as green algae and diatoms, and cyanobacteria drifting in the water.
- Benthic cyanobacteria
-
Cyanobacteria that live on sediments, rocks and other benthic organisms.
- Macroalgae
-
Macroscopic multicellular algae, such as seaweeds.
- Turf algae
-
Heterogeneous assemblages of benthic algae and cyanobacteria, visible by the naked eye but smaller than 1 cm in height.
- Carboxysomes
-
Microcompartments in cyanobacterial cells that hold the enzyme Rubisco, a key enzyme involved in the first step of CO2 fixation.
- Stokes’ law
-
A mathematical equation describing the terminal velocity of small particles in a fluid medium such as water.
- Secondary metabolites
-
Organic compounds that are produced by organisms but not directly involved in the growth or reproduction of these organisms.
- Zooplankton
-
Small animals that drift in water.
- Copepods
-
A group of small crustaceans of the subclass Copepoda, often with a cylindrical body, two large antennae and a head that is fused with the thorax.
- Cladocerans
-
A group of small crustaceans of the order Cladocera with a carapace covering the thorax and abdomen, for example, water fleas.
- β-Cyanobacteria
-
A common group of cyanobacteria with a specific type of carboxysome and Rubisco that differ from the carboxysome and Rubisco in other cyanobacteria.
- Dinoflagellates
-
A highly diverse group of unicellular photosynthetic and non-photosynthetic organisms that move through water using one longitudinal and one transverse flagellum.
- Thermocline
-
A thin layer in lakes and seas in which temperature decreases rapidly with depth, separating the warmer surface mixed layer from the colder deep water below.
- Diatoms
-
A highly diverse group of microscopically small photosynthetic algae of the class Bacillariophyceae that are enclosed by a cell wall of silica.
- Dreissenid mussels
-
Freshwater bivalve mussels of the genus Dreissena (for example, zebra and quagga mussels) indigenous to the Ponto–Caspian area and invasive species in Western Europe and North America.
- Planktivorous fish
-
Fish feeding on plankton.
- Benthivorous fish
-
Fish feeding on prey from the sediment.
- Piscivorous fish
-
Fish feeding on fish.
- Macrophytes
-
Emergent, submerged or floating aquatic plants.
Rights and permissions
About this article
Cite this article
Huisman, J., Codd, G.A., Paerl, H.W. et al. Cyanobacterial blooms. Nat Rev Microbiol 16, 471–483 (2018). https://doi.org/10.1038/s41579-018-0040-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0040-1
This article is cited by
-
Microbes and the water nexus
Nature Microbiology (2024)
-
Photosynthetically-powered phototactic active nematic liquid crystal fluids and gels
Communications Materials (2024)
-
Colonial Microcystis’ biomass affects its shift to diatom aggregates under aeration mixing
Scientific Reports (2024)
-
Long-term wetland biomonitoring highlights the differential impact of land use on macroinvertebrate diversity in Dongting Lake in China
Communications Earth & Environment (2024)
-
Cyanobacterial harmful bloom lipopolysaccharides: pro-inflammatory effects on epithelial and immune cells in vitro
Archives of Toxicology (2024)