Abstract
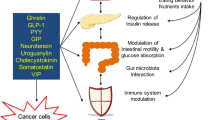
Increasing recognition of an association between obesity and many cancer types exists, but how the myriad of local and systemic effects of obesity affect key cellular and non-cellular processes within the tumour microenvironment (TME) relevant to carcinogenesis, tumour progression and response to therapies remains poorly understood. The TME is a complex cellular environment in which the tumour exists along with blood vessels, immune cells, fibroblasts, bone marrow-derived inflammatory cells, signalling molecules and the extracellular matrix. Obesity, in particular visceral obesity, might fuel the dysregulation of key pathways relevant to both the adipose microenvironment and the TME, which interact to promote carcinogenesis in at-risk epithelium. The tumour-promoting effects of obesity can occur at the local level as well as systemically via circulating inflammatory, growth factor and metabolic mediators associated with adipose tissue inflammation, as well as paracrine and autocrine effects. This Review explores key pathways linking visceral obesity and gastrointestinal cancer, including inflammation, hypoxia, altered stromal and immune cell function, energy metabolism and angiogenesis.
Key points
-
Epidemiological evidence implicates obesity as a risk factor for the development of cancers at multiple sites in the gastrointestinal tract, including the oesophagus, liver, colon, gastric cardia and pancreas.
-
Immune, metabolic and inflammation-associated properties of excess adiposity are increasingly understood, but how they influence the tumour microenvironment (TME), which comprises tumour cells, blood vessels, immune cells, fibroblasts and the extracellular matrix, remains unclear.
-
Adipocyte stem cells are increased in adipose tissue of individuals with obesity; in mouse models, these cells migrate to tumour sites and differentiate into cell types that might affect the TME.
-
Interstitial fibrosis within the TME can influence cytokine signalling, epithelial cell morphology and stem cell differentiation. Adipocyte stem cells might be a source of cancer-associated fibroblasts, and obesity might play a part in fibrosis-associated mechanosignalling within tumours.
-
Metabolic reprogramming in the TME is associated with obesity, hypoxia and angiogenesis. These processes are tightly interconnected and represent a potential target affecting not only tumours but also immune or inflammatory cells within the TME.
-
Bariatric or metabolic surgery is associated with an improvement in the metabolic profile of patients with obesity and a marked decrease in the incidence of cancer development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lauby-Secretan, B. et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med. 375, 794–798 (2016).
Kyrgiou, M. et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 356, j477 (2017).
NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 (2016).
Flegal, K. M., Kruszon-Moran, D., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315, 2284–2291 (2016).
Arnold, M. et al. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 41, 8–15 (2016).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638 (2003).
Ogden, C. L. et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 315, 2292–2299 (2016).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Ahrens, W. et al. Prevalence of overweight and obesity in European children below the age of 10. Int. J. Obes. 38, S99 (2014).
Renehan, A. G. & Soerjomataram, I. Obesity as an avoidable cause of cancer (attributable risks). Recent Results Cancer Res. 208, 243–256 (2016).
Dignam, J. J. et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J. Natl Cancer Inst. 98, 1647–1654 (2006).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Maruthur, N. M., Bolen, S., Brancati, F. L. & Clark, J. M. Obesity and mammography: a systematic review and meta-analysis. J. Gen. Intern. Med. 24, 665–677 (2009).
Maruthur, N. M., Bolen, S., Gudzune, K., Brancati, F. L. & Clark, J. M. Body mass index and colon cancer screening: a systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 21, 737–746 (2012).
Sinicrope, F. A., Foster, N. R., Sargent, D. J., O’Connell, M. J. & Rankin, C. Obesity is an independent prognostic variable in colon cancer survivors. Clin. Cancer Res. 16, 1884–1893 (2010).
Reeves, G. K. et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335, 1134 (2007).
Prizment, A. E., Flood, A., Anderson, K. E. & Folsom, A. R. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women’s Health Study. Cancer Epidemiol. Biomarkers Prev. 19, 2229–2237 (2010).
Ligibel, J. A. et al. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 32, 3568–3574 (2014).
Olson, O. C., Quail, D. F. & Joyce, J. A. Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017).
Seo, B. R. et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl Med. 7, 301ra130 (2015).
Dunlap, S. M. et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev. Res. 5, 930–942 (2012).
Santander, A. M. et al. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: the role of obesity and inflammation in breast adipose tissue. Cancers 7, 143–178 (2015).
Ackerman, D. & Simon, M. C. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 24, 472–478 (2014).
Cozzo, A. J., Fuller, A. M. & Makowski, L. Contribution of adipose tissue to development of cancer. Compr. Physiol. 8, 237–282 (2017).
Brahmkhatri, V. P., Prasanna, C. & Atreya, H. S. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed. Res. Int. 2015, 538019 (2015).
Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8, 915–928 (2008).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Vegiopoulos, A., Rohm, M. & Herzig, S. Adipose tissue: between the extremes. EMBO J. 36, 1999–2017 (2017).
Meza-Perez, S. & Randall, T. D. Immunological functions of the omentum. Trends Immunol. 38, 526–536 (2017).
Janssen, I., Katzmarzyk, P. T. & Ross, R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch. Intern. Med. 162, 2074–2079 (2002).
Klein, S. et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 15, 1061–1067 (2007).
Dong, Y. et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci. Rep. 37, BSR20170945 (2017).
Freisling, H. et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br. J. Cancer 116, 1486 (2017).
Kabir, M. et al. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 288, E454–E461 (2005).
Item, F. & Konrad, D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes. Rev. 13 (Suppl. 2), 30–39 (2012).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Yang, H. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 185, 1836–1845 (2010).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Caspar-Bauguil, S. et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 579, 3487–3492 (2005).
Perrini, S. et al. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLOS ONE 8, e57892 (2013).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Pérez-Pérez, A. et al. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 35, 71–84 (2017).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Lin, T. C. et al. Leptin signaling axis specifically associates with clinical prognosis and is multifunctional in regulating cancer progression. Oncotarget 9, 17210–17219 (2018).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Colotta, F., Allavena, P., Sica, A., Garlanda, C. & Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081 (2009).
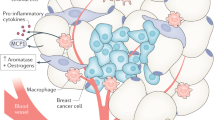
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276 (2016).
Stefan, N., Haring, H. U. & Schulze, M. B. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 6, 249–258 (2018).
Hinnouho, G. M. et al. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 36, 2294–2300 (2013).
Phillips, C. M. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann. NY Acad. Sci. 1391, 85–100 (2017).
Schmidt, F. M. et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLOS ONE 10, e0121971 (2015).
Iglesias Molli, A. E. et al. Metabolically healthy obese individuals present similar chronic inflammation level but less insulin-resistance than obese individuals with metabolic syndrome. PLOS ONE 12, e0190528 (2017).
Font-Burgada, J., Sun, B. & Karin, M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62 (2016).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Deng, B. et al. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-beta1 in gastric cancer. PLOS ONE 8, e63777 (2013).
Huber, V. et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 43, 74–89 (2017).
Anderson, K. G., Stromnes, I. M. & Greenberg, P. D. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 31, 311–325 (2017).
Amor, S. et al. Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. Int. J. Colorectal Dis. 31, 365–375 (2016).
Huang, J. et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell 33, 770–784 (2018).
Saltiel, A. R. & Olefsky, J. M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4 (2017).
Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 40, 16–28 (2014).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
D’Archivio, M. et al. ω3-PUFAs exert anti-inflammatory activity in visceral adipocytes from colorectal cancer patients. PLOS ONE 8, e77432 (2013).
Caer, C. et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 7, 3000 (2017).
Donninelli, G. et al. Distinct blood and visceral adipose tissue regulatory T cell and innate lymphocyte profiles characterize obesity and colorectal cancer. Front. Immunol. 8, 643 (2017).
Elgazar-Carmon, V., Rudich, A., Hadad, N. & Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 (2008).
Lysaght, J. et al. T lymphocyte activation in visceral adipose tissue of patients with oesophageal adenocarcinoma. Br. J. Surg. 98, 964–974 (2011).
Wouters, K. et al. Circulating classical monocytes are associated with CD11c+ macrophages in human visceral adipose tissue. Sci. Rep. 7, 42665 (2017).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Mraz, M. & Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 222, R113–R127 (2014).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Ju, C. & Tacke, F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 13, 316–327 (2016).
Kenna, T. et al. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 113, 56–63 (2004).
Toubal, A. & Lehuen, A. Lights on MAIT cells, a new immune player in liver diseases. J. Hepatol. 64, 1008–1010 (2016).
Kurioka, A., Walker, L. J., Klenerman, P. & Willberg, C. B. MAIT cells: new guardians of the liver. Clin. Transl Immunol. 5, e98 (2016).
Rohr-Udilova, N. et al. Deviations of the immune cell landscape between healthy liver and hepatocellular carcinoma. Sci. Rep. 8, 6220 (2018).
Reccia, I. et al. Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism 72, 94–108 (2017).
Narayanan, S., Surette, F. A. & Hahn, Y. S. The immune landscape in nonalcoholic steatohepatitis. Immune Netw. 16, 147–158 (2016).
Conroy, M. J. et al. Parallel profiles of inflammatory and effector memory T cells in visceral fat and liver of obesity-associated cancer patients. Inflammation 39, 1729–1736 (2016).
Ogawa, Y. et al. Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis. PLOS ONE 8, e65211 (2013).
Rensen, S. S. et al. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am. J. Pathol. 175, 1473–1482 (2009).
Ibrahim, J. et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology 143, 1061–1072 (2012).
Rau, M. et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J. Immunol. 196, 97–105 (2016).
Gadd, V. L. et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405 (2014).
Tajiri, K., Shimizu, Y., Tsuneyama, K. & Sugiyama, T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 21, 673–680 (2009).
Conroy, M. J. et al. The microenvironment of visceral adipose tissue and liver alter natural killer cell viability and function. J. Leukoc. Biol. 100, 1435–1442 (2016).
Kavanagh, M. E. et al. The esophagitis to adenocarcinoma sequence; the role of inflammation. Cancer Lett. 345, 182–189 (2014).
Cabia, B., Andrade, S., Carreira, M. C., Casanueva, F. F. & Crujeiras, A. B. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes. Rev. 17, 361–376 (2016).
Maurizi, G., Della Guardia, L., Maurizi, A. & Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 233, 88–97 (2017).
Pecht, T., Gutman-Tirosh, A., Bashan, N. & Rudich, A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev. 15, 322–337 (2014).
Galon, J. et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 232, 199–209 (2014).
Galon, J. et al. Cancer classification using the Immunoscore: a worldwide task force. J. Transl Med. 10, 205 (2012).
Pagès, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Taieb, J. et al. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: current status and future perspectives. Cancer Treat. Rev. 66, 104–113 (2018).
Strong, A. L., Burow, M. E., Gimble, J. M. & Bunnell, B. A. Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells 33, 318–326 (2015).
Strong, A. L. et al. Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int. 2017, 9216502 (2017).
Park, J., Morley, T. S., Kim, M., Clegg, D. J. & Scherer, P. E. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 10, 455–465 (2014).
Hefetz-Sela, S. & Scherer, P. E. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol. Ther. 138, 197–210 (2013).
Toren, P., Mora, B. C. & Venkateswaran, V. Diet, obesity, and cancer progression: are adipocytes the link? Lipid Insights 6, 37–45 (2013).
Freese, K. E. et al. Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res. 75, 1161–1168 (2015).
Arendt, L. M. et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 73, 6080–6093 (2013).
Eterno, V. et al. Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget 5, 613–633 (2014).
Zhao, B. C., Zhao, B., Han, J. G., Ma, H. C. & Wang, Z. J. Adipose-derived stem cells promote gastric cancer cell growth, migration and invasion through SDF-1/CXCR4 axis. Hepatogastroenterology 57, 1382–1389 (2010).
Zhang, T. et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 7, 11674 (2016).
Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O. & Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 21, 2724–2752 (2012).
Razmkhah, M., Mansourabadi, Z., Mohtasebi, M. A., Talei, A. R. & Ghaderi, A. Cancer and normal adipose-derived mesenchymal stem cells (ASCs): do they have differential effects on tumor and immune cells? Cell Biol. Int. 42, 334–343 (2017).
Pérez, L. M., Bernal, A., San Martín, N. & Gálvez, B. G. Obese-derived ASCs show impaired migration and angiogenesis properties. Arch. Physiol. Biochem. 119, 195–201 (2013).
Oñate, B. et al. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics 14, 625 (2013).
Zhang, Y. et al. Stromal cells derived from visceral and obese adipose tissue promote growth of ovarian cancers. PLOS ONE 10, e0136361 (2015).
Zhang, Y. et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 69, 5259–5266 (2009).
Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 (2004).
Zhang, Y., Bellows, C. F. & Kolonin, M. G. Adipose tissue-derived progenitor cells and cancer. World J. Stem Cells 2, 103–113 (2010).
Zhang, Y. et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 72, 5198–5208 (2012).
Jotzu, C. et al. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell. Oncol. 34, 55–67 (2011).
Bochet, L. et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582 (2016).
Nishida, T. et al. Low stromal area and high stromal microvessel density predict poor prognosis in pancreatic cancer. Pancreas 45, 593–600 (2016).
Spaeth, E. L. et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLOS ONE 4, e4992 (2009).
Underwood, T. J. et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J. Pathol. 235, 466–477 (2015).
Huijbers, A. et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann. Oncol. 24, 179–185 (2013).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85 (2012).
Erez, N., Truitt, M., Olson, P. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Zhu, Q. et al. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 5, e1295 (2014).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420 (2009).
Zheng, X. et al. EMT program is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525 (2015).
Fischer, K. R. et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472 (2015).
Brabletz, T., Kalluri, R., Nieto, M. A. & Weinberg, R. A. EMT in cancer. Nat. Rev. Cancer 18, 128 (2018).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178 (2014).
De Craene, B. & Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110 (2013).
Li, L. & Li, W. Epithelial–mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol. Ther. 150, 33–46 (2015).
Ricciardi, M. et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br. J. Cancer 112, 1067 (2015).
Uthaya Kumar, D. B. et al. TLR4 signaling via NANOG cooperates with STAT3 to activate Twist1 and promote formation of tumor-initiating stem-like cells in livers of mice. Gastroenterology 150, 707–719 (2015).
Allott, E. H. et al. Elevated tumor expression of PAI-1 and SNAI2 in obese esophageal adenocarcinoma patients and impact on prognosis. Clin. Transl Gastroenterol. 3, e12 (2012).
Allott, E. H. et al. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol. Carcinog. 52, 144–154 (2013).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Martin, F. et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 124, 317–326 (2010).
Shinagawa, K. et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int. J. Cancer 127, 2323–2333 (2010).
Kabashima-Niibe, A. et al. Mesenchymal stem cells regulate epithelial–mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 104, 157–164 (2013).
Strong, A. L. et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 17, 1–16 (2015).
Yao-Borengasser, A. et al. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol. Rep. 33, 2689–2694 (2015).
Suarez-Carmona, M., Lesage, J., Cataldo, D. & Gilles, C. EMT and inflammation: inseparable actors of cancer progression. Mol. Oncol. 11, 805–823 (2017).
Clark, A. G. & Vignjevic, D. M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 36, 13–22 (2015).
Friedl, P. & Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362 (2003).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Qiao, L. et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 18, 500 (2008).
Lu, Y. R. et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol. Ther. 7, 245–251 (2008).
Ohlsson, L. B., Varas, L., Kjellman, C., Edvardsen, K. & Lindvall, M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 75, 248–255 (2003).
Cousin, B. et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLOS ONE 4, e6278 (2009).
Wu, X.-B. et al. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR-mediated NF-κB activation. Sci. Rep. 6, 21420 (2016).
Guaita-Esteruelas, S., Guma, J., Masana, L. & Borras, J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol. Cell Endocrinol. 462, 107–118 (2018).
Dirat, B. et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465 (2011).
Trevellin, E. et al. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 6, 11203–11215 (2015).
Rebours, V. et al. Obesity and fatty pancreatic infiltration are risk factors for pancreatic precancerous lesions (PanIN). Clin. Cancer Res. 21, 3522–3528 (2015).
Peverill, W., Powell, L. W. & Skoien, R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int. J. Mol. Sci. 15, 8591–8638 (2014).
Yu, J., Shen, J., Sun, T. T., Zhang, X. & Wong, N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin. Cancer Biol. 23, 483–491 (2013).
Poli, G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol. Aspects Med. 21, 49–98 (2000).
Wang, Z., Li, Z., Ye, Y., Xie, L. & Li, W. Oxidative stress and liver cancer: etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016, 7891574 (2016).
Malaguarnera, M., Di Rosa, M., Nicoletti, F. & Malaguarnera, L. Molecular mechanisms involved in NAFLD progression. J. Mol. Med. 87, 679–695 (2009).
Chen, J. S. et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol. Res. 39, 177–186 (2009).
Mullen, M. & Gonzalez-Perez, R. R. Leptin-induced JAK/STAT signaling and cancer growth. Vaccines 4, 26 (2016).
Tsochatzis, E., Papatheodoridis, G. V. & Archimandritis, A. J. The evolving role of leptin and adiponectin in chronic liver diseases. Am. J. Gastroenterol. 101, 2629–2640 (2006).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 64, 60–78 (2015).
Nepal, S. & Park, P. H. Modulation of cell death and survival by adipokines in the liver. Biol. Pharm. Bull. 38, 961–965 (2015).
He, G. et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17, 286–297 (2010).
Menon, S. et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci. Signal. 5, ra24 (2012).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Di Caro, S. et al. Role of body composition and metabolic profile in Barrett’s oesophagus and progression to cancer. Eur. J. Gastroenterol. Hepatol. 28, 251–260 (2016).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Moschen, A. R. et al. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 17, 840–845 (2011).
Kern, P. A., Ranganathan, S., Li, C., Wood, L. & Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E745–E751 (2001).
Divella, R., De Luca, R., Abbate, I., Naglieri, E. & Daniele, A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 7, 2346–2359 (2016).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
O’Sullivan, K. E., Reynolds, J. V., O’Hanlon, C., O’Sullivan, J. N. & Lysaght, J. Could signal transducer and activator of transcription 3 be a therapeutic target in obesity-related gastrointestinal malignancy? J. Gastrointest. Cancer 45, 1–11 (2014).
Chang, W. J., Du, Y., Zhao, X., Ma, L. Y. & Cao, G. W. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 20, 4586–4596 (2014).
Ericksen, R. E. et al. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut 63, 385–394 (2014).
Galizia, G. et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin. Immunol. 102, 169–178 (2002).
Knupfer, H. & Preiss, R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int. J. Colorectal Dis. 25, 135–140 (2010).
Voronov, E. & Apte, R. N. IL-1 in colon inflammation, colon carcinogenesis and invasiveness of colon cancer. Cancer Microenviron. 8, 187–200 (2015).
Lysaght, J. et al. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett. 312, 62–72 (2011).
Rausch, L. K., Netzer, N. C., Hoegel, J. & Pramsohler, S. The linkage between breast cancer, hypoxia, and adipose tissue. Front. Oncol. 7, 211 (2017).
Trayhurn, P., Wang, B. & Wood, I. S. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 100, 227–235 (2008).
Ratushnyy, A., Lobanova, M. & Buravkova, L. B. Expansion of adipose tissue-derived stromal cells at “physiologic” hypoxia attenuates replicative senescence. Cell Biochem. Funct. 35, 232–243 (2017).
Trayhurn, P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu. Rev. Nutr. 34, 207–236 (2014).
Kang, Y. E. et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLOS ONE 11, e0154003 (2016).
Rasouli, N. Adipose tissue hypoxia and insulin resistance. J. Investig. Med. 64, 830–832 (2016).
Weljie, A. M. & Jirik, F. R. Hypoxia-induced metabolic shifts in cancer cells: moving beyond the Warburg effect. Int. J. Biochem. Cell Biol. 43, 981–989 (2011).
van Uden, P., Kenneth, N. S. & Rocha, S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem. J. 412, 477–484 (2008).
Bakirtzi, K. et al. The neurotensin-HIF-1α-VEGFα axis orchestrates hypoxia, colonic inflammation, and intestinal angiogenesis. Am. J. Pathol. 184, 3405–3414 (2014).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Baba, Y. et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am. J. Pathol. 176, 2292–2301 (2010).
Lin, D. & Wu, J. Hypoxia inducible factor in hepatocellular carcinoma: atherapeutic target. World J. Gastroenterol. 21, 12171–12178 (2015).
Dai, X. et al. Association of PD-L1 and HIF-1α coexpression with poor prognosis in hepatocellular carcinoma. Transl Oncol. 11, 559–566 (2018).
Catalan, V. et al. IL-32α-induced inflammation constitutes a link between obesity and colon cancer. Oncoimmunology 6, e1328338 (2017).
Yang, Y. et al. Dysregulation of over-expressed IL-32 in colorectal cancer induces metastasis. World J. Surg. Oncol. 13, 146 (2015).
Wincewicz, A., Sulkowska, M., Koda, M. & Sulkowski, S. Clinicopathological significance and linkage of the distribution of HIF-1α and GLUT-1 in human primary colorectal cancer. Pathol. Oncol. Res. 13, 15–20 (2007).
Lolmede, K., Durand de Saint Front, V., Galitzky, J., Lafontan, M. & Bouloumie, A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. Relat. Metab. Disord. 27, 1187–1195 (2003).
Zheng, H. et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 26, 3579–3583 (2006).
Lemoine, A. Y., Ledoux, S. & Larger, E. Adipose tissue angiogenesis in obesity. Thromb. Haemost. 110, 661–668 (2013).
Neels, J. G., Thinnes, T. & Loskutoff, D. J. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 18, 983–985 (2004).
Fukumura, D. et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 93, e88–e97 (2003).
Miyazawa-Hoshimoto, S. et al. Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am. J. Physiol. Endocrinol. Metab. 288, E1128–E1136 (2005).
Engin, A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: hypoxia hypothesis. Adv. Exp. Med. Biol. 960, 305–326 (2017).
Tam, J. et al. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLOS ONE 4, e4974 (2009).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Incio, J. et al. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumor progression in obesity. Clin. Cancer Res. 22, 2993–3004 (2016).
Anagnostoulis, S. et al. Human leptin induces angiogenesis in vivo. Cytokine 42, 353–357 (2008).
Bouloumie, A., Drexler, H. C., Lafontan, M. & Busse, R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 83, 1059–1066 (1998).
Koda, M. et al. Expression of the obesity hormone leptin and its receptor correlates with hypoxia-inducible factor-1α in human colorectal cancer. Ann. Oncol. 18 (Suppl. 6), 116–119 (2007).
Koda, M., Sulkowska, M., Kanczuga-Koda, L., Surmacz, E. & Sulkowski, S. Overexpression of the obesity hormone leptin in human colorectal cancer. J. Clin. Pathol. 60, 902–906 (2007).
Howard, J. M. et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br. J. Surg. 97, 1020–1027 (2010).
Zhang, C. et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun. 4, 2935 (2013).
Epstein, T., Gatenby, R. A. & Brown, J. S. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLOS ONE 12, e0185085 (2017).
Paspulati, R. M. & Gupta, A. PET/MR imaging in cancers of the gastrointestinal tract. PET Clin. 11, 403–423 (2016).
Pisarsky, L. et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 15, 1161–1174 (2016).
Nakajima, E. C. & Van Houten, B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol. Carcinog. 52, 329–337 (2013).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8, 275–283 (2007).
Phelan, J. J. et al. Examining the connectivity between different cellular processes in the Barrett tissue microenvironment. Cancer Lett. 371, 334–346 (2016).
Huang, D., Li, C. & Zhang, H. Hypoxia and cancer cell metabolism. Acta Biochim. Biophys. Sin. 46, 214–219 (2014).
Johnson, A. R., Milner, J. J. & Makowski, L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 249, 218–238 (2012).
Krzywinska, E. & Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 6, 56 (2018).
Lynam-Lennon, N. et al. Excess visceral adiposity induces alterations in mitochondrial function and energy metabolism in esophageal adenocarcinoma. BMC Cancer 14, 907 (2014).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-L1 in immune cells and tumors. Front. Immunol. 8, 1300 (2017).
Ikhlas, S. & Ahmad, M. Metformin: insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 185, 53–62 (2017).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63, 2985–3023 (2014).
LeBlanc, E. S., O’Connor, E., Whitlock, E. P., Patnode, C. D. & Kapka, T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the US Preventive Services Task Force. Ann. Intern. Med. 155, 434–447 (2011).
Colquitt, J. L., Pickett, K., Loveman, E. & Frampton, G. K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 8, CD003641 (2014).
Gloy, V. L. et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347, f5934 (2013).
Reges, O. et al. Association of bariatric surgery using laparoscopic banding, roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy versus usual care obesity management with all-cause mortality. JAMA 319, 279–290 (2018).
Sjöström, L. et al. Bariatric surgery and long-term cardiovascular events. JAMA 307, 56–65 (2012).
Sjöström, L. et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 10, 653–662 (2009).
Adams, T. D. et al. Cancer incidence and mortality after gastric bypass surgery. Obesity 17, 796–802 (2009).
Schauer, D. P. et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann. Surg. https://doi.org/10.1097/SLA.0000000000002525 (2017).
Campbell, K. L., Landells, C. E., Fan, J. & Brenner, D. R. A systematic review of the effect of lifestyle interventions on adipose tissue gene expression: implications for carcinogenesis. Obesity 25 (Suppl. 2), 40–51 (2017).
Bai, Y. & Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 16, 127–136 (2015).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Pendyala, S., Neff, L. M., Suarez-Farinas, M. & Holt, P. R. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am. J. Clin. Nutr. 93, 234–242 (2011).
Todoric, J. et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 49, 2109–2119 (2006).
Rowan, B. G. et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLOS ONE 9, e89595 (2014).
Orecchioni, S. et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 73, 5880–5891 (2013).
Bellows, C. F., Zhang, Y., Chen, J., Frazier, M. L. & Kolonin, M. G. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol. Biomarkers Prev. 20, 2461–2468 (2011).
Baglioni, S. et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLOS ONE 7, e36569 (2012).
Author information
Authors and Affiliations
Contributions
J.O., J.L., C.L.D. and J.V.R. researched data for the article. All authors contributed equally to the discussion of content, writing of the article and the reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Sullivan, J., Lysaght, J., Donohoe, C.L. et al. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol 15, 699–714 (2018). https://doi.org/10.1038/s41575-018-0069-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0069-7
This article is cited by
-
Dormancy of cutaneous melanoma
Cancer Cell International (2024)
-
Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review
Journal of Translational Medicine (2024)
-
Extracellular vesicle-mediated ferroptosis, pyroptosis, and necroptosis: potential clinical applications in cancer therapy
Cell Death Discovery (2024)
-
Deficiency of histone variant macroH2A1.1 is associated with sexually dimorphic obesity in mice
Scientific Reports (2023)
-
Modulation of rectal cancer stemness, patient outcome and therapy response by adipokines
Journal of Physiology and Biochemistry (2023)