Abstract
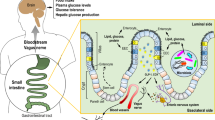
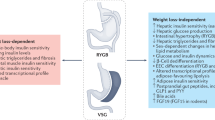
Metabolic surgery is the best treatment for long-term weight loss maintenance and comorbidity control. Metabolic operations were originally intended to change anatomy to alter behaviour, but we now understand that the anatomical changes can modulate physiology to change behaviour. They are no longer considered only mechanically restrictive and/or malabsorptive procedures; rather, they are considered metabolic procedures involving complex physiological changes, whereby gut adaptation influences signalling pathways in several other organs, including the liver and the brain, regulating hunger, satiation, satiety, body weight, glucose metabolism and immune functions. The integrative physiology of gut adaptation after these operations consists of a complex mechanistic web of communication between gut hormones, bile acids, gut microbiota, the brain and both enteric and central nervous systems. The understanding of nutrient sensing via enteroendocrine cells, the enteric nervous system, hypothalamic peptides and adipose tissue and of the role of inflammation has advanced our knowledge of this integrative physiology. In this Review, we focus on the adaptation of gut physiology to the anatomical alterations from Roux-en-Y gastric bypass and vertical sleeve gastrectomy and the influence of these procedures on food intake, weight loss, nonalcoholic fatty liver disease (NAFLD) and cancer. We also aim to demonstrate the underlying mechanisms that could explain how metabolic surgery could be used as a therapeutic option in NAFLD and certain obesity-related cancers.
Key points
-
Gut adaptation after metabolic surgery is pivotal in facilitating weight loss and comorbidity improvement.
-
The complex interactions between the gut–brain–endocrine axis, the gut microbiota and bile acid kinetics are postulated to have a role in reducing food intake and improving metabolic control after metabolic surgery.
-
The integrative physiology of changes in eating behaviour, weight loss, gut hormones, bile acids, gut microbiota and adipocyte-derived factors is also postulated to result in comorbidity improvement.
-
Further research on the mechanistic effects of metabolic surgery on nonalcoholic fatty liver disease and cancer are vital before metabolic surgery can be recommended as a treatment for these conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flum, D. R. et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 361, 445–454 (2009).
Buchwald, H. Metabolic surgery (Grune and Stratton, New York, 1978).
O’Brien, P. E., McPhail, T., Chaston, T. B. & Dixon, J. B. Systematic review of medium-term weight loss after bariatric operations. Obes. Surg. 16, 1032–1040 (2006).
Brethauer, S. A., Hammel, J. P. & Schauer, P. R. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg. Obes. Relat. Dis. 5, 469–475 (2009).
Wolnerhanssen, B. et al. Enteroendocrine cell population is reduced in obesity and restored after sleeve gastrectomy (LSG). Br. J. Surg. 102, 10 (2015).
Mumphrey, M. B., Patterson, L. M., Zheng, H. & Berthoud, H. R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 25, e70–e79 (2013).
le Roux, C. W. et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann. Surg. 252, 50–56 (2010).
Nora, M., Morais, T., Almeida, R., Guimaraes, M. & Monteiro, M. P. Should Roux-en-Y gastric bypass biliopancreatic limb length be tailored to achieve improved diabetes outcomes? Med. (Baltimore) 96, e8859 (2017).
Pournaras, D. J. et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes. Surg. 20, 56–60 (2010).
Park, M. Y. et al. Gut microbiota-associated bile acid deconjugation accelerates hepatic steatosis in ob/ob mice. J. Appl. Microbiol. 121, 800–810 (2016).
Zheng, X. et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 15, 120 (2017).
Begley, M., Gahan, C. G. M. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
Bose, M. et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J. Diabetes 2, 47–55 (2009).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238.
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Latorre, R., Sternini, C., De Giorgio, R. & Greenwood-Van Meerveld, B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 28, 620–630 (2016).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Richards, P. et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 (2014).
de Heuvel, E., Wallace, L., Sharkey, K. A. & Sigalet, D. L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-gamma signaling. Am. J. Physiol. Endocrinol. Metab. 303, E994–E1005 (2012).
Patterson, L. M., Zheng, H. & Berthoud, H. R. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat. Rec 266, 10–20 (2002).
Nakagawa, A. et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 110, 36–43 (2004).
Seeley, R. J., Schwartz, M. W., Porte, D., Woods, S. C. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Le Roux, C. W. et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 243, 108–114 (2006).
Theodorakis, M. J. et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 290, E550–E559 (2006).
Group, T. L. A. R. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369, 145–154 (2013).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Hofmann, W., Guido, M.v. K., Stroebe, W., Ramanathan, S. & Aarts, H. As pleasure unfolds: hedonic responses to tempting food. Psychol. Sci. 21, 1863–1870 (2010).
Miras, A. D. et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 96, 467–473 (2012).
Wilson-Pérez, H. E. et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. 37, 288–295 (2013).
Bjorklund, P., Lonroth, H. & Fandriks, L. Manometry of the upper gut following roux-en-y gastric bypass indicates that the gastric pouch and roux limb act as a common cavity. Obes. Surg. 25, 1833–1841 (2015).
Kolakowski, S. Jr., Kirkland, M. L. & Schuricht, A. L. Routine postoperative upper gastrointestinal series after Roux-en-Y gastric bypass: determination of whether it is necessary. Arch. Surg. 142, 930–934; discussion 934 (2007).
Vigneshwaran, B. et al. Impact of sleeve gastrectomy on type 2 diabetes mellitus, gastric emptying time, glucagon-like peptide 1 (GLP-1), ghrelin and leptin in non-morbidly obese subjects with BMI 30–35.0 kg/m2: a prospective study. Obes. Surg. 26, 2817–2823 (2016).
Sista, F. et al. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg. Obes. Relat. Dis. 13, 7–14 (2017).
Nikkie van der, W. et al. Cross-species comparison of genes related to nutrient sensing mechanisms expressed along the intestine. PLoS ONE 9, e107531 (2014).
Dirksen, C. et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol. Motil. 25, 346–e255 (2013).
Parker, H. E. et al. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 165, 414–423 (2012).
Roberts, R. E. et al. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin. Endocrinol. 74, 67–72 (2011).
Shah, S. et al. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg. Obes. Relat. Dis. 6, 152–157 (2010).
Dirksen, C. et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 37, 1452–1459 (2013).
le Roux, C. W. et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 246, 780–785 (2007).
Goldstone, A. P. et al. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J. Clin. Endocrinol. Metab. 101, 599–609 (2016).
Pournaras, D. J. et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg. Obes. Relat. Dis. 8, 371–374 (2012).
Neary, N. M. et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology 146, 5120–5127 (2005).
Day, J. W. et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 (2009).
Chandarana, K. et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60, 810–818 (2011).
Werling, M. et al. Preoperative assessment of gut hormones does not correlate to weight loss after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 10, 822–828 (2014).
Wilson-Perez, H. E. et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62, 2380–2385 (2013).
Chambers, A. P. et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol. Behav. 105, 120–123 (2011).
Svane, M. S. et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 40, 1699–1706 (2016).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Buhmann, H., le Roux, C. W. & Bueter, M. The gut–brain axis in obesity. Best Pract. Res. Clin. Gastroenterol. 28, 559–571 (2014).
Dorland, W. A. N. Dorland’s illustrated medical dictionary (Elsevier Saunders, Philadelphia, PA, 2012).
Soret, R. et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138, 1772–1782 (2010).
Neunlist, M. et al. Glycine activates myenteric neurones in adult guinea-pigs. J. Physiol. 536, 727–739 (2001).
Liu, M., Seino, S. & Kirchgessner, A. L. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J. Neurosci. 19, 10305–10317 (1999).
Kiciak, A., Wolinski, J., Borycka, K., Zabielski, R. & Bielecki, K. Roux-en-Y or ‘uncut’ Roux procedure? Relation of intestinal migrating motor complex recovery to the preservation of the network of interstitial cells of Cajal in pigs. Exp. Physiol. 92, 399–408 (2007).
Reichardt, F., Krueger, D. & Schemann, M. Leptin excites enteric neurons of guinea-pig submucous and myenteric plexus. Neurogastroenterol. Motil. 23, e165–e170 (2011).
Slattery, J. A., Page, A. J., Dorian, C. L., Brierley, S. M. & Blackshaw, L. A. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J. Physiol. 577, 295–306 (2006).
Poole, D. P. et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol. Motil. 22, 814–825 (2010).
Berthoud, H.-R. The vagus nerve, food intake and obesity. Regulatory Pept. 149, 15–25 (2008).
Iggo, A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q. J. Exp. Physiol. Cogn. Med. Sci. 42, 398–409 (1957).
Berthoud, H. R., Kressel, M., Raybould, H. E. & Neuhuber, W. L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol. 191, 203–212 (1995).
Fu-Cheng, X. et al. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflügers Arch. 433, 571–579 (1997).
Elliott, R. M. et al. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 138, 159–166 (1993).
Plamboeck, A. et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1117–1127 (2013).
Berthoud, H.-R., Lynn, P. A. & Blackshaw, L. A. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, 1371–1381 (2001).
Phillips, R. J. & Powley, T. L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Brain Res. Rev. 34, 1–26 (2000).
Zagorodnyuk, V. P., Chen, B. N. & Simon, J. H. B. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534, 255–268 (2001).
Kral, J. G., Gortz, L., Hermansson, G. & Wallin, G. S. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J. Surg. 17, 75–78 (1993).
Ikramuddin, S. et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA 312, 915–922 (2014).
le Roux, C. W. et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 90, 4521–4524 (2005).
Peters, J. H. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J. Comp. Neuro. 521, 3584–3599 (2013).
Hao, Z. et al. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes. Surg. 24, 2145–2151 (2014).
Ballsmider, L. A. et al. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015, 601985 (2015).
Okafor, P. N. et al. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes. Res. Clin. Pract. 9, 274–280 (2015).
Qvigstad, E. et al. A novel technique of Roux-en-Y gastric bypass reversal for postprandial hyperinsulinemic hypoglycaemia: a case report. Int. J. Surg. Case Rep. 21, 91–94 (2016).
Liu, T. et al. Effects of sleeve gastrectomy plus trunk vagotomy compared with sleeve gastrectomy on glucose metabolism in diabetic rats. World J. Gastroenterol. 23, 3269–3278 (2017).
Norgren, R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3, 207–218 (1978).
Mullier, A., Bouret, S. G., Prevot, V. & Dehouck, B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 518, 943–962 (2010).
Ciofi, P. et al. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology 150, 5509–5519 (2009).
Rodríguez, E. M., Blázquez, J. L. & Guerra, M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31, 757–776 (2010).
Norsted, E., Gömüç, B. & Meister, B. Protein components of the blood–brain barrier (BBB) in the mediobasal hypothalamus. J. Chem. Neuroanat. 36, 107–121 (2008).
Bewick, G. A. et al. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) and agouti-related protein (AgRP) neurons coexpress the NOP1 receptor and nociceptin alters CART and AgRP release. Endocrinology 146, 3526–3534 (2005).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Xu, A. W. et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 115, 951–958 (2005).
Larsen, P. J., Tang-Christensen, M., Holst, J. J. & Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270 (1997).
Batterham, R. L. et al. Inhibition of food intake in obese subjects by peptide YY3–36. N. Engl. J. Med. 349, 941–948 (2003).
Koda, S. et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 146, 2369–2375 (2005).
Batterham, R. L. et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418, 650–654 (2002).
Schwartz, M. W. et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46, 2119–2123 (1997).
Tao, Y. X. The Melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr. Rev. 31, 506–543 (2010).
Bonnefond, A. et al. Eating behavior, low-frequency functional mutations in the melanocortin-4 receptor (MC4R) gene, and outcomes of bariatric operations: a 6-year prospective study. Diabetes Care 39, 1384–1392 (2016).
Jelin, E. B. et al. Melanocortin-4 receptor signaling is not required for short-term weight loss after sleeve gastrectomy in pediatric patients. Int. J. Obes. 40, 550–553 (2016).
Sogin, M. L. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. USA 106, 2365–2370 (2009).
Furet, J.-P. et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057 (2010).
Murphy, R. et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes. Surg. 27, 917–925 (2017).
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 5, 178ra141 (2013).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009).
Tazoe, H. et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 30, 149–156 (2009).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Nohr, M. K. et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells versus FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154, 3552–3564 (2013).
Tulika Arora, F. S. et al. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 11, 2035–2046 (2017).
Pournaras, D. J. et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153, 3613–3619 (2012).
Stefater, M. A. et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141, 939–949 (2011).
Kohli, R. et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J. Clin. Endocrinol. Metab. 98, E708–E712 (2013).
Patti, M. E. et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17, 1671–1677 (2009).
Risstad, H. et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg. Obes. Relat. Dis. 13, 1544–1553 (2017).
Brighton, C. A. et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156, 3961–3970 (2015).
Katsuma, S., Hirasawa, A. & Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329, 386–390 (2005).
Ryan, K. K. et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–183 (2014).
Holt, J. A. et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17, 1581–1591 (2003).
Jansen, P. L. et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig. Dis. 29, 48–51 (2011).
Fu, L. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004).
Keitel, V. et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 (2010).
Fukiya, S. et al. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol. Lett. 293, 263–270 (2009).
Pournaras, D. J. & le Roux, C. W. Are bile acids the new gut hormones? Lessons from weight loss surgery models. Endocrinology 154, 2255–2256 (2013).
Neff, K. J. et al. Beyond weight loss: evaluating the multiple benefits of bariatric surgery after Roux-en-Y gastric bypass and adjustable gastric band. Obes. Surg. 24, 684–691 (2014).
Biemann, R. et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: results of a randomized controlled trial. Sci. Rep. 6, 30173 (2016).
Watanabe, M. et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 (2004).
Sayiner, M., Koenig, A., Henry, L. & Younossi, Z. M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin. Liver Dis. 20, 205–214 (2016).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348, 1625–1638 (2003).
Setiawan, V. W. et al. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology 64, 1969–1977 (2016).
Promrat, K. et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51, 121–129 (2010).
Harrison, S. A., Fecht, W., Brunt, E. M. & Neuschwander-Tetri, B. A. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 49, 80–86 (2009).
Bowman, T. A. et al. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology 151, 5157–5164 (2010).
Lassailly, G. et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 149, 379–388 (2015).
Praveen Raj, P. et al. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: “NASHOST” prospective observational trial. Surg. Obes. Relat. Dis. 11, 1315–1322 (2015).
Tai, C. M. et al. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 22, 1016–1021 (2012).
Yu, H., Jia, W. & Guo, Z. K. Reducing liver fat by low carbohydrate caloric restriction targets hepatic glucose production in non-diabetic obese adults with non-alcoholic fatty liver disease. J. Clin. Med. 3, 1050–1063 (2014).
Petersen, K. F. et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54, 603–608 (2005).
Bell, L. N. et al. Bariatric surgery-induced weight loss reduces hepatic lipid peroxidation levels and affects hepatic cytochrome P-450 protein content. Ann. Surg. 251, 1041–1048 (2010).
Pournaras, D. J. et al. Improved glucose metabolism after gastric bypass: evolution of the paradigm. Surg. Obes. Relat. Dis. 12, 1457–1465 (2016).
Al-Mrabeh, A., Hollingsworth, K. G., Steven, S. & Taylor, R. Morphology of the pancreas in type 2 diabetes: effect of weight loss with or without normalisation of insulin secretory capacity. Diabetologia 59, 1753–1759 (2016).
Yamashita, H. et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl Acad. Sci. USA 98, 9116–9121 (2001).
Musso, G., Gambino, R., Pacini, G., De Michieli, F. & Cassader, M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am. J. Clin. Nutr. 89, 558–567 (2009).
Nomura, K. & Yamanouchi, T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 23, 203–208 (2012).
le Roux, C. W. et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1057–1066 (2011).
Russell-Jones, D. & Gough, S. Recent advances in incretin-based therapies. Clin. Endocrinol. 77, 489–499 (2012).
Pachikian, B. D. et al. Prebiotic approach alleviates hepatic steatosis: Implication of fatty acid oxidative and cholesterol synthesis pathways. Mol. Nutr. Food Res. 57, 347–359 (2013).
Svegliati-Baroni, G. et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 31, 1285–1297 (2011).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Bechmann, L. P. et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57, 1394–1406 (2013).
Zhang, S., Wang, J., Liu, Q. & Harnish, D. C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51, 380–388 (2009).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Bhutta, H. Y. et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS ONE 10, e0122273 (2015).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Potthoff, Matthew,J. et al. FGF15/19 Regulates Hepatic Glucose Metabolism by Inhibiting the CREB-PGC-1α Pathway. Cell Metab. 13, 729–738 (2011).
Haluzíková, D. et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects: FGF-19 and -21 after sleeve gastrectomy. Obesity 21, 1335–1342 (2013).
Myronovych, A. et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity 22, 2301–2311 (2014).
Lau, E., Carvalho, D. & Freitas, P. Gut microbiota: association with NAFLD and metabolic disturbances. BioMed Res. Int. 2015, 979515–979519 (2015).
Spruss, A. et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50, 1094–1104 (2009).
Sarkola, T. & Eriksson, C. J. Effect of 4-methylpyrazole on endogenous plasma ethanol and methanol levels in humans. Alcohol Clin. Exp. Res. 25, 513–516 (2001).
Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 65, 278–290 (2006).
da Costa, K. A., Garner, S. C., Chang, J. & Zeisel, S. H. Effects of prolonged (1 year) choline deficiency and subsequent re-feeding of choline on 1,2-sn-diradylglycerol, fatty acids and protein kinase C in rat liver. Carcinogenesis 16, 327–334 (1995).
Spencer, M. D. et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140, 976–986 (2011).
Exley, M. A., Hand, L., O’Shea, D. & Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 223, R41–R48 (2014).
Bugianesi, E. Non-alcoholic steatohepatitis and cancer. Clin. Liver Dis. 11, 191–207 (2007).
Galanakis, C. G. et al. Computed tomography-based assessment of abdominal adiposity changes and their impact on metabolic alterations following bariatric surgery. World J. Surg. 39, 417–423 (2015).
Moschen, A. R. et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Gut 59, 1259–1264 (2010).
Adachi, M. & Brenner, D. A. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47, 677–685 (2008).
Schaffler, A., Scholmerich, J. & Buchler, C. Mechanisms of disease: adipocytokines and visceral adipose tissue — emerging role in nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 273–280 (2005).
Kamada, Y. et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125, 1796–1807 (2003).
Gressner, O. A. et al. Connective tissue growth factor is a Smad2 regulated amplifier of transforming growth factor β actions in hepatocytes — but without modulating bone morphogenetic protein 7 signaling. Hepatology 49, 2021–2030 (2009).
Xu, X. J. et al. Improved insulin sensitivity 3 months after RYGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes 64, 3155–3159 (2015).
Salminen, A., Hyttinen, J. M. T. & Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 89, 667–676 (2011).
Basen-Engquist, K. & Chang, M. Obesity and cancer risk: recent review and evidence. Curr. Oncol. Rep. 13, 71–76 (2011).
Eliassen, A. H., Colditz, G. A., Rosner, B., Willett, W. C. & Hankinson, S. E. Adult weight change and risk of postmenopausal breast cancer. JAMA 296, 193–201 (2006).
Nagle, C. M. et al. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. Eur. J. Cancer 49, 2717–2726 (2013).
Chlebowski, R. T. et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the women’s intervention nutrition study. J. Natl Cancer Institute 98, 1767–1776 (2006).
Pierce, J. P. et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) Randomized Trial. JAMA 298, 289–298 (2007).
Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357, 753–761 (2007).
Sjöström, L. et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet. Oncol. 10, 653–662 (2009).
Anveden, Å. et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol. Oncol. 145, 224–229 (2016).
Blouin, K. et al. Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am. J. Physiol. Endocrinol. Metab. 296, E244–E255 (2009).
Kaaks, R. & Calle, E. E. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Legro, R. S. et al. Effects of gastric bypass surgery on female reproductive function. J. Clin. Endocrinol. Metab. 97, 4540–4548 (2012).
Bastard, J.-P. et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Network 17, 4–12 (2006).
Moulin, C. M., Marguti, I., Peron, J. P., Halpern, A. & Rizzo, L. V. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes. Surg. 21, 112–118 (2011).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Hicks, B. M. et al. Glucagon-like peptide-1 analogues and risk of breast cancer in women with type 2 diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMJ 355 (2016).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Bjerre Knudsen, L. et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151, 1473–1486 (2010).
Egan, A. G. et al. Pancreatic safety of incretin-based drugs — FDA and EMA assessment. N. Engl. J. Med. 370, 794–797 (2014).
Trevisan, M. et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol. Biomarkers Prev. 10, 937–941 (2001).
Yamada, T., De Souza, A. T., Finkelstein, S. & Jirtle, R. L. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc. Natl Acad. Sci. USA 94, 10351–10355 (1997).
Chen, F., Beezhold, K. & Castranova, V. JNK1, a potential therapeutic target for hepatocellular carcinoma. Biochim. Biophys. Acta 1796, 242–251 (2009).
Ignacio Barrasa, J. et al. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis 16, 1054–1067 (2011).
Tao, W. et al. Colorectal cancer prognosis following obesity surgery in a population-based cohort study. Obes. Surg. 27, 1233–1239 (2017).
Lax, S. et al. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int. J. Cancer 130, 2232–2239 (2012).
Cao, W. et al. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G322–327 (2013).
Hong, J. et al. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut 59, 170–180 (2010).
Casaburi, I. et al. Chenodeoxycholic acid through a TGR5-dependent CREB signaling activation enhances cyclin D1 expression and promotes human endometrial cancer cell proliferation. Cell Cycle 11, 2699–2710 (2012).
Yang, J. I. et al. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem. Biophys. Res. Commun. 361, 156–161 (2007).
Chen, M. C., Chen, Y. L., Wang, T. W., Hsu, H. P. & Lai, M. D. Membrane bile acid receptor TGR5 predicts good prognosis in ampullary adenocarcinoma patients with hyperbilirubinemia. Oncol. Rep. 36, 1997–2008 (2016).
Phelan, J. P., Reen, F. J., Dunphy, N., O’Connor, R. & O’Gara, F. Bile acids destabilise HIF-1α and promote anti-tumour phenotypes in cancer cell models. BMC Cancer 16, 476 (2016).
Lee, W. S. et al. Ursodeoxycholic acid induces death receptor-mediated apoptosis in prostate cancer cells. J. Cancer Prev. 22, 16–21 (2017).
Miyoshi, Y. et al. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 9, 5699–5704 (2003).
Cust, A. E. et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 92, 255–263 (2007).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Phillips, S. A. et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52, 667–674 (2003).
Hancke, K., Grubeck, D., Hauser, N., Kreienberg, R. & Weiss, J. M. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res. Treat. 119, 367–367 (2010).
Cao, L. et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142, 52–64 (2010).
Brakenhielm, E. et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl Acad. Sci. USA 101, 2476–2481 (2004).
Liu, Y. et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 16, 847–857 (2009).
Kiraly, O., Gong, G., Olipitz, W., Muthupalani, S. & Engelward, B. P. Inflammation-induced cell proliferation potentiates dna damage-induced mutations in vivo. PLoS Genet 11, e1004901 (2015).
Vázquez, L. A. et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J. Clin. Endocrinol. Metabolism 90, 316–322 (2005).
Uzun, H. et al. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes. Surg. 14, 659–665 (2004).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Ohsugi, M. et al. CD8 + effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Bertola, A. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247 (2012).
Strissel, K. J. et al. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity 18, 1918–1925 (2010).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Kisakol, G. et al. Effect of surgical weight loss on free radical and antioxidant balance: a preliminary report. Obes. Surg. 12, 795–800; discussion 800–801 (2002).
Lynch, L. et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 24, 510–519 (2016).
Mingrone, G. et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 366, 1577–1585 (2012).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N. Engl. J. Med. 376, 641–651 (2017).
Sjostrom, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Sjöström, L. et al. Bariatric surgery and long-term cardiovascular events. JAMA 307, 56–65 (2012).
Buchwald, H. et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 292, 1724–1737 (2004).
Asztalos, B. F. et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J. Lipid Res. 51, 2405–2412 (2010).
Haines, K. L. et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery 141, 354–358 (2007).
Sjostrom, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Karlsson, J., Taft, C., Ryden, A., Sjostrom, L. & Sullivan, M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int. J. Obes. 31, 1248–1261 (2007).
Date, Y. et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141, 4255–4261 (2000).
Lawrence, C. B., Snape, A. C., Baudoin, F. M. & Luckman, S. M. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143, 155–162 (2002).
Tymitz, K., Engel, A., McDonough, S., Hendy, M. P. & Kerlakian, G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes. Surg. 21, 125–130 (2011).
Cummings, D. E. et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 346, 1623–1630 (2002).
Cote, C. D., Zadeh-Tahmasebi, M., Rasmussen, B. A., Duca, F. A. & Lam, T. K. T. Hormonal signaling in the gut. J. Biol. Chem. 289, 11642–11649 (2014).
Kellum, J. M. et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann. Surg. 211, 763–770 (1990).
Rubino, F. et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann. Surg. 240, 236–242 (2004).
Jacobsen, S. H. et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and β-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 22, 1084–1096 (2012).
Peterli, R. et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes. Surg. 22, 740–748 (2012).
Mallipedhi, A. et al. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 10, 860–869 (2014).
Moran-Atkin, E., Brody, F., Fu, S. W. & Rojkind, M. Changes in GIP gene expression following bariatric surgery. Surg. Endosc. 27, 2492–2497 (2013).
Svane, M. S. et al. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 310, E505–E514 (2016).
Dube, P. E. & Brubaker, P. L. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm. Metab. Res. 36, 755–760 (2004).
Romero, F. et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc. 26, 2231–2239 (2012).
Sloth, B., Holst, J. J., Flint, A., Gregersen, N. T. & Astrup, A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. Endocrinol. Metab. 292, E1062–E1068 (2007).
Vazquez-Vela, M. E., Torres, N. & Tovar, A. R. White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 39, 715–728 (2008).
Bays, H. E. et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: executive summary. J. Clin. Lipidol 10, 15–32 (2016).
Bays, H. E. et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J. Clin. Lipidol 7, 304–383 (2013).
Vijgen, G. H. et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 97, E1229–E1233 (2012).
Lo, K. A. & Sun, L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci. Rep. 33, e00065 (2013).
Acknowledgements
C.W.L. acknowledges funding from Science Foundation Ireland (ref 12/YI/B2480) and the Health Research Board (USIRL-2016-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.W.L. is part of advisory boards for Ethicon Endo-surgery, GI Dynamics, Herbalife and Novo Nordisk. P.S. and D.J.B. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sinclair, P., Brennan, D.J. & le Roux, C.W. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol 15, 606–624 (2018). https://doi.org/10.1038/s41575-018-0057-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0057-y
This article is cited by
-
Diffusion tensor imaging findings in the hunger and satiety centers of the brain after bariatric surgery: a preliminary study
Irish Journal of Medical Science (1971 -) (2024)
-
Hypoabsorptive surgeries cause limb-dependent changes in the gut endocannabinoidome and microbiome in association with beneficial metabolic effects
International Journal of Obesity (2023)
-
Famsin, a novel gut-secreted hormone, contributes to metabolic adaptations to fasting via binding to its receptor OLFR796
Cell Research (2023)
-
Mechanisms linking bariatric surgery to adipose tissue, glucose metabolism, fatty liver disease and gut microbiota
Langenbeck's Archives of Surgery (2023)
-
Medikamentöse Therapie der Adipositas – Konkurrenz zur bariatrischen Chirurgie oder sinnvolle Ergänzung?
Die Chirurgie (2023)