Abstract
In humans, the thyroid hormones T3 and T4 are synthesized in the thyroid gland in a process that crucially involves the iodoglycoprotein thyroglobulin. The overall structure of thyroglobulin is conserved in all vertebrates. Upon thyroglobulin delivery from thyrocytes to the follicular lumen of the thyroid gland via the secretory pathway, multiple tyrosine residues can become iodinated to form mono-iodotyrosine (MIT) and/or di-iodotyrosine (DIT); however, selective tyrosine residues lead to preferential formation of T4 and T3 at distinct sites. T4 formation involves oxidative coupling between two DIT side chains, and de novo T3 formation involves coupling between an MIT donor and a DIT acceptor. Thyroid hormone synthesis is stimulated by TSH activating its receptor (TSHR), which upregulates the activity of many thyroid gene products involved in hormonogenesis. Additionally, TSH regulates post-translational changes in thyroglobulin that selectively enhance its capacity for T3 formation — this process is important in iodide deficiency and in Graves disease. 167 different mutations, many of which are newly discovered, are now known to exist in TG (encoding human thyroglobulin) that can lead to defective thyroid hormone synthesis, resulting in congenital hypothyroidism.
Key points
The first definitive evidence of a complete TG gene appears with the development of the vertebrates, and once appearing in evolution, the entire structure of thyroglobulin, as well as its ability to be secreted, has been retained thereafter.
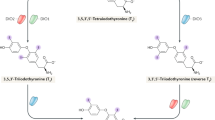
The synthesis of T3 and T4 within thyroglobulin involves oxidative coupling between iodinated tyrosine residues on thyroglobulin.
The main T3-forming site within thyroglobulin couples a mono-iodotyrosine donor at the antepenultimate residue of one monomer with a di-iodotyrosine acceptor in the same residue of the apposed monomer within a dimer.
Post-translational modifications of thyroglobulin include phosphorylation for which the secretory pathway kinase FAM20C has been implicated.
TSH stimulation of thyrocytes promotes post-translational modifications that can alter thyroglobulin structure in a way that favours T3 formation upon iodination, whereas defects in TSH-mediated stimulation result in thyroglobulin with diminished capacity to form T3.
167 TG mutations exist that can cause congenital hypothyroidism; although the disease is usually inherited as an autosomal recessive trait, patients with congenital hypothyroidism bearing monoallelic mutations of TG have recently been reported.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tovo-Neto, A., da Silva Rodrigues, M., Habibi, H. R. & Nóbrega, R. H. Thyroid hormone actions on male reproductive system of teleost fish. Gen. Comp. Endocrinol. 265, 230–236 (2018).
Saito, M., Yamasu, K. & Suyemitsu, T. Binding properties of thyroxine to nuclear extract from sea urchin larvae. Zoolog. Sci. 29, 79–82 (2012).
Hodin, J. Expanding networks: Signaling components in and a hypothesis for the evolution of metamorphosis. Integr. Comp. Biol. 46, 719–742 (2006).
Heyland, A., Reitzel, A. M. & Hodin, J. Thyroid hormones determine developmental mode in sand dollars (Echinodermata: Echinoidea). Evol. Dev. 6, 382–392 (2004).
Saito, M. et al. Induction of metamorphosis in the sand dollar Peronella japonica by thyroid hormones. Dev. Growth Differ. 40, 307–312 (1998).
Laudet, V. The origins and evolution of vertebrate metamorphosis. Curr. Biol. 21, R726–R737 (2011).
Dardente, H., Hazlerigg, D. G. & Ebling, F. J. P. Thyroid hormone and seasonal rhythmicity. Front. Endocrinol. 5, 1–11 (2014).
Mullur, R., Liu, Y.-Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382 (2014).
Kouidhi, S. & Clerget-Froidevaux, M.-S. Integrating thyroid hormone signaling in hypothalamic control of metabolism: crosstalk between nuclear receptors. Int. J. Mol. Sci. 19, 1–20 (2018).
Nicoloff, J. T., Low, J. C., Dussault, J. H. & Fisher, D. A. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J. Clin. Invest. 51, 473–483 (1972).
Kalderon, B., Hertz, R. & Bar-Tana, J. Effect of thyroid hormone treatment on redox and phosphate potentials in rat liver. Endocrinology 131, 400–407 (1992).
Venditti, P. & Di Meo, S. Thyroid hormone-induced oxidative stress. Cell. Mol. Life Sci. 63, 414–434 (2006).
Nagayama, Y. et al. Regulation of thyroid peroxidase and thyroglobulin gene expression by thyrotropin in cultured human thyroid cells. J. Clin. Endocrinol. Metab. 68, 1155–1159 (1989).
Kang, H. S. et al. GLIS3 is indispensable for TSH/TSHR-dependent thyroid hormone biosynthesis and follicular cell proliferation. J. Clin. Invest. 127, 4326–4337 (2017).
Köhrle, J. in Thyroid Hormone Nuclear Receptor Methods and Protocols (eds Plateroti, M. & Samarut, J.) 85–104 (Springer New York, 2018).
Cary, G. A., Cameron, A. R. & Hinman, V. F. EchinoBase: tools for echinoderm genome analyses. Methods Mol. Biol. 1757, 349–369 (2018).
Kinjo, S., Kiyomoto, M., Yamamoto, T., Ikeo, K. & Yaguchi, S. HpBase: a genome database of a sea urchin, Hemicentrotus pulcherrimus. Dev. Growth Differ. 60, 174–182 (2018).
Paris, M. & Laudet, V. The history of a developmental stage: metamorphosis in chordates. Genesis 46, 657–672 (2008).
Paris, M., Brunet, F., Markov, G., Schubert, M. & Laudet, V. The amphioxus genome enlightens the evolution of the thyroid hormone signaling pathway. Dev. Genes Evol. 218, 667–680 (2008).
Holland, L. Z. et al. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 18, 1100–1111 (2008).
Putnam, N. H. et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1–7 (2008).
Paris, M. et al. Active metabolism of thyroid hormone during metamorphosis of amphioxus. Integr. Comp. Biol. 50, 63–74 (2010).
Ogasawara, M. & Satoh, N. Isolation and characterization of endostyle-specific genes in the Ascidian Ciona intestinalis. Biol. Bull. 195, 60–69 (1998).
Kimura, S. Thyroid-specific enhancer-binding protein: role in thyroid function and organogenesis. Trends Endocrinol. Metab. 7, 247–252 (1996).
Monaco, F., Dominici, R., Andreoli, M., Pirro, R. D. E. & Roche, J. Thyroid hormone formation in thyroglobulin synthesized in the Amphioxus (Branchiostoma lanceolatum pallas). Comp. Biochem. Physiol. B 70, 341–343 (1981). This paper demonstrates, within the amphioxus endostyle, the synthesis of thyroid hormone in a large protein that has properties similar to those of thyroglobulin, despite complete genome sequencing subsequently confirming the absence of a vertebrate-style TG gene in this organism.
Targovnik, H. M. in Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text (eds Braverman, L. E. & Cooper, D.) 74–92 (Lippincott Williams & Wilkins, 2012). This comprehensive review describes the structure and function of thyroglobulin and TG and discusses mutations that cause congenital hypothyroidism.
Holzer, G. et al. Thyroglobulin represents a novel molecular architecture of vertebrates. J. Biol. Chem. 291, 16553–16566 (2016). This paper confirms the presence of TG in lamprey and Xenopus and indicates functional conservation in thyroid hormone synthesis throughout all vertebrates.
Smith, J. J. et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 45, 415–421 (2013).
Youson, J. H. Is lamprey metamorphosis regulated by thyroid hormones? Am. Zool. 37, 441–460 (1997).
Kluge, B., Renault, N. & Rohr, K. B. Anatomical and molecular reinvestigation of lamprey endostyle development provides new insight into thyroid gland evolution. Dev. Genes Evol. 215, 32–40 (2005).
Malthiéry, Y. & Lissitzky, S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur. J. Biochem. 165, 491–498 (1987). This paper determines the complete cDNA nucleotide sequence of thyroglobulin, thereby establishing that the region-specific domain structure of human thyroglobulin is comparable to that in other vertebrate species.
Matos, L. P. L. et al. Regulation of thyroid sodium-iodide symporter in different stages of goiter: possible involvement of reactive oxygen species. Clin. Exp. Pharmacol. Physiol. 12, 3218–3221 (2017).
Benvenga, S. & Guarneri, F. Homology of pendrin, sodium-iodide symporter and apical iodide transporter. Front. Biosci. 23, 1864–1873 (2018).
Silveira, J. C. & Kopp, P. A. Pendrin and anoctamin as mediators of apical iodide efflux in thyroid cells. Curr. Opin. Endocrinol. Diabetes Obes. 22, 374–380 (2015).
Fong, P. Thyroid iodide efflux: a team effort? J. Physiol. 589, 5929–5939 (2011).
Van Den Hove, M. F. et al. The loss of the chloride channel, ClC-5, delays apical iodide efflux and induces a euthyroid goiter in the mouse thyroid gland. Endocrinology 147, 1287–1296 (2006).
Portulano, C., Paroder-Belenitsky, M. & Carrasco, N. The Na+/I- symporter (NIS): mechanism and medical impact. Endocr. Rev. 35, 106–149 (2014).
Rhoden, K. J., Cianchetta, S., Duchi, S. & Romeo, G. Fluorescence quantitation of thyrocyte iodide accumulation with the yellow fluorescent protein variant YFP-H148Q/I152L. Anal. Biochem. 373, 239–246 (2008).
Belforte, F. S. et al. Kinetic characterization of human thyroperoxidase. Normal and pathological enzyme expression in Baculovirus System: a molecular model of functional expression. Mol. Cell. Endocrinol. 404, 9–15 (2015).
Saber-Lichtenberg, Y. et al. Covalent cross-linking of secreted bovine thyroglobulin by transglutaminase. FASEB J. 14, 1005–1014 (2000).
Muzza, M. & Fugazzola, L. Disorders of H2O2 generation. Best Pract. Res. Clin. Endocrinol. Metab. 31, 225–240 (2017).
De Deken, X., Corvilain, B., Dumont, J. E. & Miot, F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid. Redox Signal. 20, 2776–2793 (2014).
Lisi, S. et al. Defective thyroglobulin storage in LDL receptor-associated protein-deficient mice. Am. J. Physiol. Cell Physiol. 290, C1160–C1167 (2006).
Marinò, M. et al. Role of thyroglobulin endocytic pathways in the control of thyroid hormone release. Am. J. Physiol. Cell Physiol. 279, C1295–C1306 (2000).
Delom, F., Mallet, B., Carayon, P. & Lejeune, P. Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein. J. Biol. Chem. 276, 21337–21342 (2001).
Berndorfer, U., Wilms, H. & Herzog, V. Multimerization of thyroglobulin (TG) during extracellular storage: isolation of highly cross-linked TG from human thyroids. J. Clin. Endocrinol. Metab. 81, 1918–1926 (1996).
Leonardi, A. et al. Presence of dityrosine bridges in thyroglobulin and their relationship with iodination. Biochem. Biophys. Res. Commun. 202, 38–43 (1994).
Baudry, N. et al. Dityrosine bridge formation and thyroid hormone synthesis are tightly linked and are both dependent on N-glycans. FEBS Lett. 396, 223–226 (1996).
Taurog, A., Dorris, M. L. & Lamas, L. Comparison of lactoperoxidase- and thyroid peroxidase-catalyzed iodination and coupling. Endocrinology 94, 1286–1294 (1974).
Lamas, L. & Taurog, A. The importance of thyroglobulin structure in thyroid peroxidase-catalyzed conversion of diiodotyrosine to thyroxine. Endocrinology 100, 1129–1136 (1977). This paper shows that the native structure of thyroglobulin is critical to the efficiency of the hormonogenic coupling reaction.
Dunn, J. T. & Dunn, A. D. Update on intrathyroidal iodine metabolism. Thyroid 11, 407–414 (2001).
Brix, K. & Lemansky, P. H. V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology 137, 1963–1974 (1996).
Rubio, I. G. S. & Medeiros-Neto, G. Mutations of the thyroglobulin gene and its relevance to thyroid disorders. Curr. Opin. Endocrinol. Diabetes. Obes. 16, 373–378 (2009).
Mascia, A. et al. Rab7 regulates CDH1 endocytosis, circular dorsal ruffles genesis, and thyroglobulin internalization in a thyroid cell line. J. Cell. Physiol. 231, 1695–1708 (2016).
Croizet-Berger, K., Daumerie, C., Couvreur, M., Courtoy, P. J. & van den Hove, M.-F. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc. Natl Acad. Sci. USA 99, 8277–8282 (2002).
Brix, K., Linke, M., Tepel, C. & Herzog, V. Cysteine proteinases mediate extracellular prohormone processing in the thyroid. Biol. Chem. 382, 717–725 (2001).
Jordans, S. et al. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 10, 23 (2009).
Linke, M., Jordans, S., Mach, L., Herzog, V. & Brix, K. Thyroid stimulating hormone upregulates secretion of cathepsin B from thyroid epithelial cells. Biol. Chem. 383, 773–784 (2002).
Suban, D. et al. Cathepsin C and plasma glutamate carboxypeptidase secreted from Fischer rat thyroid cells liberate thyroxin from the N-terminus of thyroglobulin. Biochimie 94, 719–726 (2012).
Lisi, S. et al. Preferential megalin-mediated transcytosis of low-hormonogenic thyroglobulin: a control mechanism for thyroid hormone release. Proc. Natl Acad. Sci. USA 100, 14858–14863 (2003).
Marino, M., Zheng, G. & Mccluskey, R. T. Megalin (gp330) is an endocytic receptor for thyroglobulin on cultured fisher rat thyroid cells. J. Biol. Chem. 274, 12898–12904 (1999).
Miquelis, R. et al. The N-acetylglucosamine-specific receptor of the thyroid. Binding characteristics, partial characterization, and potential role. J. Biol. Chem. 262, 15291–15298 (1987).
Miquelis, R. et al. Intracellular routing of GLcNAc-bearing molecules in thyrocytes: selective recycling through the Golgi Apparatus Raymond. J. Cell Biol. 123, 1695–1706 (1993).
Pacifico, F., Liguoro, D., Acquaviva, R., Formisano, S. & Consiglio, E. Thyroglobulin binding and TSH regulation of the RHL-1 subunit of the asialoglycoprotein receptor in rat thyroid. Biochimie 81, 493–496 (1999).
Montuori, N. et al. The rat asialoglycoprotein receptor binds the amino-terminal domain of thyroglobulin. Biochem. Biophys. Res. Commun. 268, 42–46 (2000).
Mezghrani, A. et al. Protein-disulfide Isomerase (PDI) in FRTL5 Cells. J. Biol. Chem. 275, 1920–1929 (2000).
Friedrichs, B. et al. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Invest. 111, 1733–1745 (2003).
Oda, K. et al. Follicular thyroglobulin induces cathepsin H expression and activity in thyrocytes. Biochem. Biophys. Res. Commun. 483, 541–546 (2017).
Kopp, P. in Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text (eds Braverman, L. E. & Cooper, D.) 48–74 (Lippincott Williams & Wilkins, 2012).
van de Graaf, S. a et al. Up to date with human thyroglobulin. J. Endocrinol. 170, 307–321 (2001).
Müller, J. et al. Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology 155, 315–325 (2018).
Krause, G. & Hinz, K. M. Thyroid hormone transport across L-type amino acid transporters: What can molecular modelling tell us? Mol. Cell. Endocrinol. 458, 68–75 (2017).
Hinz, K. M. et al. Structural insights into thyroid hormone transport mechanisms of the L-type amino acid transporter 2. Mol. Endocrinol. 29, 933–942 (2015).
Mayerl, S. et al. Thyroid hormone transporters MCT8 and OATP1C1 control skeletal muscle regeneration. Stem Cell Rep. 10, 1959–1974 (2018).
Weber, J. et al. Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland. Eur. J. Cell Biol. 96, 440–456 (2017). This paper suggests that lysosomal degradation of thyroglobulin and thyroid hormone transporter proteins in the thyroid gland are physiologically regulated.
Gnidehou, S. et al. Cloning and characterization of a novel isoform of iodotyrosine dehalogenase 1 (DEHAL1) DEHAL1C from human thyroid: comparisons with DEHAL1 and DEHAL1B. Thyroid 16, 715–724 (2006).
Gnidehou, S. et al. Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site. FASEB J. 18, 1574–1576 (2004).
Renko, K. et al. A nonradioactive DEHAL assay for testing substrates, inhibitors, and monitoring endogenous activity. Endocrinology 157, 4516–4525 (2018).
Gavaret, J., Cahnmann, H. J. & Nunez, J. Thyroid hormone synthesis in thyroglobulin. J. Biol. Chem. 256, 9167–9173 (1981).
Chanoine, J. P. et al. The thyroid gland is a major source of circulating T3 in the rat. J. Clin. Invest. 91, 2709–2713 (1993).
Larsen, P. R. Thyroidal triiodothyronine and thyroxine in Graves’ disease: correlation with presurgical treatment, thyroid status, and iodine content. J. Clin. Endocrinol. Metab. 41, 1098–1104 (1975).
Schneider, M. J. et al. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 15, 2137–2148 (2001).
Schneider, M. J. et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147, 580–589 (2006).
Galton, V. A., Schneider, M. J., Clark, A. S. & St. Germain, D. L. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′-deiodinases. Endocrinology 150, 2957–2963 (2009). This paper reveals normal serum T 3 levels in mice with whole-body double knockout of DIO1 and DIO2 deiodinases, indicating robust T 3 formation in the absence of deiodination of T 4.
Bianco, A. C. & Larsen, P. R. Cellular and structural biology of the deiodinases. Thyroid 15, 777–786 (2005).
Lavado-Autric, R. et al. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology 154, 529–536 (2013).
Citterio, C. E. et al. De novo triiodothyronine formation from thyrocytes activated by Thyroid Stimulating Hormone. J. Biol. Chem. 292, 15434–15444 (2017). This paper demonstrates increased de novo T 3 formation in thyroglobulin secreted from TSH-stimulated thyrocytes and suggests that thyroglobulin phosphorylation may contribute to this effect.
Sellitti, D. F. & Suzuki, K. Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 24, 625–638 (2014).
Di Jeso, B. & Arvan, P. Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr. Rev. 37, 2–36 (2016).
Dentice, M., Cordeddu, V., Rosica, A. & Macchia, P. E. Missense mutation in the transcription factor NKX2–5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J. Clin. Endocrinol. Metab. 91, 1428–1433 (2005).
Ma, R., Morshed, S. A., Latif, R. & Davies, T. F. TAZ induction directs differentiation of thyroid follicular cells from human embryonic stem cells. Thyroid 27, 292–299 (2017).
Lacroix, L. et al. HEX, PAX-8 and TTF-1 gene expression in human thyroid tissues: a comparative analysis with other genes involved in iodide metabolism. Clin. Endocrinol. 64, 398–404 (2006).
Pellizzari, L. et al. Expression and function of the homeodomain-containing protein Hex in thyroid cells. Nucleic Acids Res. 28, 2503–2511 (2000).
Berg, V., Vassart, G. & Christophe, D. Identification of a thyroid-specific and cAMP-responsive enhancer in the upstream sequences of the human thyroglobulin promoter. Biochim. Biophys. Acta 1307, 35–38 (1996).
Berg, V., Vassart, G. & Christophe, D. A zinc-dependent DNA-binding activity co-operates with cAMP-responsive-element-binding protein to activate the human thyroglobulin enhancer. Biochem. J. 323, 349–357 (1997).
Baas, F., van Ommen, G. J., Bikker, H., Arnberg, A. C. & de Vijlder, J. J. The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb. Nucleic Acids Res. 14, 5171–5186 (1986).
Meijerink, P. H. et al. The gene for the human Src-like adaptor protein (hSLAP) is located within the 64-kb intron of the thyroglobulin gene. Eur. J. Biochem. 254, 297–303 (1998).
Lee, J. & Arvan, P. Repeat motif-containing regions within thyroglobulin. J. Biol. Chem. 286, 26327–26333 (2011).
Veneziani, B. M., Giallauria, F. & Gentile, F. The disulfide bond pattern between fragments obtained by the limited proteolysis of bovine thyroglobulin. Biochimie 81, 517–525 (1999).
Molina, F., Bouanani, M., Pau, B. & Granier, C. Characterization of the type-1 repeat from thyroglobulin, a cysteine-rich module found in proteins from different families. Eur. J. Biochem. 240, 125–133 (1996).
Cousin, X. et al. The alpha/beta fold family of proteins database and the cholinesterase gene server ESTHER. Nucleic Acids Res. 25, 143–146 (1997).
Targovnik, H. M., Citterio, C. E. & Rivolta, C. M. Thyroglobulin gene mutations in congenital hypothyroidism. Horm. Res. Paediatr. 75, 311–321 (2011).
De Jaco, A., Dubi, N., Camp, S. & Taylor, P. Congenital hypothyroidism mutations affect common folding and trafficking in the α/β-hydrolase fold proteins. FEBS J. 279, 4293–4305 (2012).
Lee, J., Di jeso, B. & Arvan, P. The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone. J. Clin. Invest. 118, 2950–2958 (2008). This paper demonstrates that the thyroglobulin ChEL domain can function independently to assist in the folding stability, intracellular transport and secretion of the upstream regions of thyroglobulin.
Park, Y.-N. & Arvan, P. The acetylcholinesterase homology region is essential for normal conformational maturation and secretion of thyroglobulin. J. Biol. Chem. 279, 17085–17089 (2004).
Lee, J., Di Jeso, B. & Arvan, P. Maturation of thyroglobulin protein region I. J. Biol. Chem. 286, 33045–33052 (2011).
Kim, P. S. & Arvant, P. Folding and assembly of newly synthesized thyroglobulin occurs in a pre-Golgi compartment. J. Biol. Chem. 266, 12412–12418 (1991).
Suzuki, K. et al. Thyroglobulin autoregulation of thyroid-specific gene expression and follicular function. Rev. Endocr. Metab. Disord. 1, 217–224 (2000).
Lee, J., Wang, X., Di Jeso, B. & Arvan, P. The cholinesterase-like domain, essential in thyroglobulin trafficking for thyroid hormone synthesis, is required for protein dimerization. J. Biol. Chem. 284, 12752–12761 (2009).
Citterio, C. E., Morishita, Y., Dakka, N., Veluswamy, B. & Arvan, P. Relationship between the dimerization of thyroglobulin and its ability to form triiodothyronine. J. Biol. Chem. 293, 4860–4869 (2018). This paper confirms that the major T 3-forming site of thyroglobulin involves MIT–DIT coupling of the antepenultimate residues of the two thyroglobulin monomers within the thyroglobulin dimer.
Desruisseau, S., Franc, J. L., Gruffat, D. & Chabaud, O. Glycosylation of thyroglobulin secreted by porcine cells cultured in chamber system: thyrotropin controls the number of oligosaccharides and their anionic residues. Endocrinology 134, 1676–1684 (1994).
Desruisseau, S., Valette, A., Franc, J. L. & Chabaud, O. Thyrotropin controls dolichol-linked sugar pools and oligosaccharyltransferase activity in thyroid cells. Mol. Cell. Endocrinol. 122, 223–228 (1996).
Di Jeso, B. et al. Modulation of the carbohydrate moiety of thyroglobulin by thyrotropin and calcium in fisher rat thyroid line-5. J. Biol. Chem. 267, 1938–1944 (1992).
Grollman, E. F., Saji, M., Shimura, Y., Lau, J. T. & Ashwell, G. Thyrotropin regulation of sialic acid expression in rat thyroid cells. J. Biol. Chem. 268, 3604–3609 (1993).
Nlend, M. C., Cauvi, D., Venot, N., Desruisseau, S. & Chabaud, O. Thyrotropin regulates tyrosine sulfation of thyroglobulin. Eur. J. Endocrinol. 141, 61–69 (1999).
Haeberli, A., Kneubuehl, F. & Studer, H. Changes in the polypeptide assembly of guinea pig thyroglobulin induced by thyrotropin-regulated thyroid activity. Endocrinology 109, 523–529 (1981).
Consiglio, E. et al. Characterization of phosphate residues on thyroglobulin. J. Biol. Chem. 262, 10304–10314 (1987).
Yang, S., Pollock, H. G. & Rawitch, A. B. Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin 1. Arch. Biochem. Biophys. 327, 61–70 (1996).
MacPhee-Quigley, K., Vedvick, T. S., Taylor, P. & Taylor, S. S. Profile of the disulfide bonds in acetylcholinesterase. J. Biol. Chem. 261, 13565–13570 (1986).
Di Jeso, B. et al. Mixed-disulfide folding intermediates between thyroglobulin and endoplasmic reticulum resident oxidoreductases ERp57 and protein disulfide isomerase. Mol. Cell. Biol. 25, 9793–9805 (2005).
Di Jeso, B. et al. Transient covalent interactions of newly synthesized thyroglobulin with oxidoreductases of the endoplasmic reticulum. J. Biol. Chem. 289, 11488–11496 (2014). This paper identifies several distinct chaperone and oxidoreductase partners that engage newly synthesized thyroglobulin during its early folding in the ER.
Di Jeso, B. et al. Folding of thyroglobulin in the calnexin/calreticulin pathway and its alteration by loss of Ca2+ from the endoplasmic reticulum. Biochem. J. 370, 449–458 (2003).
Jessop, C. E. et al. ERp57 is essential for efficient folding of glycoproteins sharing common structural domains. EMBO J. 26, 28–40 (2007).
Vali, M., Rose, N. R. & Caturegli, P. Thyroglobulin as autoantigen: structure-function relationships. Rev. Endocr. Metab. Disord. 1, 69–77 (2000).
Xavier, A. C. W., Maciel, R. M. B., Vieira, J. G. H., Dias-da-Silva, M. R. & Martins, J. R. M. Insights into the posttranslational structural heterogeneity of thyroglobulin and its role in the development, diagnosis, and management of benign and malignant thyroid diseases. Arch. Endocrinol. Metab. 60, 66–75 (2016).
Mallet, B., Lejeune, P., Baudry, N., Niccoli, P. & Carayon, P. N-glycans modulate in vivo and in vitro thyroid hormone synthesis. J. Biol. Chem. 270, 29881–29888 (1995).
Conte, M. et al. A single chondroitin 6-sulfate oligosaccharide unit at Ser-2730 of human thyroglobulin enhances hormone formation and limits proteolytic accessibility at the carboxyl terminus. Potential insights into thyroid homeostasis and autoimmunity. J. Biol. Chem. 281, 22200–22211 (2006).
Tsuji, T., Yamam, Irimura, T. & Osawa, T. Structure of carbohydrate unit A of porcine thyroglobulin. Biochem. J. 195, 691–699 (1981).
Yamamoto, K., Tsuji, T., Irimura, T. & Osawa, T. The structure of carbohydrate unit B of porcine thyroglobulin. Biochem. J. 195, 701–713 (1981).
Spiro, R. G. & Bhoyroo, V. D. Occurrence of sulfate in the asparagine-linked complex carbohydrate units of thyroglobulin. Identification and localization of galactose 3-sulfate and N-acetylglucosamine 6-sulfate residues in the human and calf proteins. J. Biol. Chem. 263, 14351–14358 (1988).
Zhao, J., Song, E., Zhu, R. & Mechref, Y. Parallel data acquisition of in-source fragmented glycopeptides to sequence the glycosylation sites of proteins. Electrophoresis 37, 1420–1430 (2016).
Tassi, V., Liguoro, D., Consiglio, E. & Acquaviva, A. in Advances in Post-Translational Modifications of Proteins and Aging (eds Zappia, V., Galletti, P., Porta, R. & Wold, F.) 541–549 (Springer US, 1988).
Wen, G., Ringseis, R. & Eder, K. Endoplasmic reticulum stress inhibits expression of genes involved in thyroid hormone synthesis and their key transcriptional regulators in FRTL-5 thyrocytes. PLOS ONE 12, e0187561 (2017).
Tagliabracci, V. S. et al. Secreted kinase phosphorylated extracellular proteins that regulate biomineralization. Science 336, 1150–1153 (2012).
Mertins, P. et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 (2016).
Cauvi, D., Venot, N., Nlend, M.-C. & Chabaud, O. M. Thyrotropin and iodide regulate sulfate concentration in thyroid cells. Relationship to thyroglobulin sulfation. Can. J. Physiol. Pharmacol. 81, 1131–1138 (2003).
Schneider, A. B., Mccurdy, A., Chang, T., Dudlak, D. & Magner, J. Metabolic labeling of human thyroglobulin with [35S]sulfate: incorporation into chondroitin 6-sulfate and endoglycosidase-F-susceptible carbohydrate units. Endocrinology 122, 2428–2435 (1988).
Schneider, A. B. & Dudlak, D. Chondroitin chain and complex carbohydrate chains of human thyroglobulin: studies in normal and neoplastic thyroid tissue. Endocrinology 124, 356–362 (1989).
Sakurai, S., Fogelfeld, L., Ries, A. & Schneider, A. B. Anionic complex-carbohydrate units of human thyroglobulin. Endocrinology 127, 2056–2063 (1990).
Chambard, M. et al. Thyrotrophin regulation of apical and basal exocytosis of thyroglobulin by porcine thyroid monolayers. J. Mol. Endocrinol. 4, 193–199 (1990).
Nlend, M. C., Cauvi, D., Venot, N. & Chabaud, O. Sulfated tyrosines of thyroglobulin are involved in thyroid hormone synthesis. Biochem. Biophys. Res. Commun. 262, 193–197 (1999).
Venot, N., Nlend, M. C., Cauvi, D. & Chabaud, O. The hormonogenic tyrosine 5 of porcine thyroglobulin is sulfated. Biochem. Biophys. Res. Commun. 298, 193–197 (2002).
Nlend, M. C., Cauvi, D. M., Venot, N. & Chabaud, O. Role of sulfated tyrosines of thyroglobulin in thyroid hormonosynthesis. Endocrinology 146, 4834–4843 (2005).
Palumbo, G., Gentile, F., Condorelli, G. L. & Salvatore, G. The earliest site of iodination in thyroglobulin is residue number 5. J. Biol. Chem. 265, 8887–8892 (1990). This paper shows that thyroglobulin Tyr5 becomes iodinated before other tyrosines, suggesting its exposure on the surface of the 3D molecular structure of thyroglobulin.
Dedieu, A., Gaillard, J.-C., Pourcher, T., Darrouzet, E. & Armengaud, J. Revisiting iodination sites in thyroglobulin with an organ-oriented shotgun strategy. J. Biol. Chem. 286, 259–269 (2011).
Dunn, J. T. & Dunn, A. D. The importance of thyroglobulin structure for thyroid hormone biosynthesis. Biochimie 81, 505–509 (1999). This comprehensive review summarizes the main hormonogenic sites of thyroglobulin that are involved in T 4 and T 3 formation.
Lamas, L., Anderson, P., Fox, J. W. & Dunn, J. T. Consensus sequences for early iodination and hormonogenesis in human thyroglobulin. J. Biol. Chem. 264, 13541–13545 (1989).
Izumi, M. & Larsen, P. R. Triiodothyronine, thyroxine, and iodine in purified thyroglobulin from patients with Graves’ disease. J. Clin. Invest. 59, 1105–1112 (1977).
Dunn, A. D., Corsi, C. M., Myers, H. E. & Dunn, J. T. Tyrosine 130 is an important outer ring donor for thyroxine formation in thyroglobulin. J. Biol. Chem. 273, 25223–25229 (1998). This paper provides independent evidence in support of earlier work suggesting that DIT at residue 130 of thyroglobulin is the donor to the acceptor DIT at residue 5.
Marriq, C., Lejeune, P. J., Venot, N. & Vinet, L. Hormone formation in the isolated fragment 1–171 of human thyroglobulin involves the couple tyrosine 5 and tyrosine 130. Mol. Cell. Endocrinol. 81, 155–164 (1991).
Ohmiya, Y., Hayashi, H. & Kondo, T. K. Y. Location of dehydroalanine bovine thyroglobulin residues in the amino acid sequence of bovine thyroglobulin. J. Biol. Chem. 265, 9066–9071 (1990).
Cetrangolo, G. P. et al. Hormonogenic donor Tyr2522 of bovine thyroglobulin. Insight into preferential T3 formation at thyroglobulin carboxyl terminus at low iodination level. Biochem. Biophys. Res. Commun. 450, 488–493 (2014). This paper presents strong mass spectrometry evidence for efficient mono-iodination and di-iodination of the antepenultimate residue that is engaged in T 3 synthesis in thyroglobulin.
Mallet, B. et al. Tyrosine iodination and iodotyrosyl coupling of the N-terminal thyroid hormone forming site of human thyroglobulin modulate its binding to auto- and monoclonal antibodies. Mol. Cell. Endocrinol. 88, 89–95 (1992).
den Hartog, M. T., Sijmons, C. C., Bakker, O., Ris-Stalpers, C. & de Vijlder, J. J. Importance of the content and localization of tyrosine residues for thyroxine formation within the N-terminal part of human thyroglobulin. Eur. J. Endocrinol. 132, 611–617 (1995).
Gentile, F., Ferranti, P., Mamone, G., Malorni, A. & Salvatore, G. Identification of hormonogenic tyrosines in fragment 1218–1591 of bovine thyroglobulin by mass spectrometry. Biochemistry 272, 639–646 (1997).
Marsili, A., Zavacki, A. M., Harney, J. W. & Larsen, P. R. Physiological role and regulation of iodothyronine deiodinases: a 2011 update. J. Endocrinol. Invest. 34, 395–407 (2011).
Laugwitz, K. L. et al. The human thyrotropin receptor: A heptahelical receptor capable of stimulating members of all four G protein families. Proc. Natl Acad. Sci. USA 93, 116–120 (1996).
Maenhaut, C., Brabant, G., Vassart, G. & Dumont, J. E. In vitro and in vivo regulation of thyrotropin receptor mRNA levels in dog and human thyroid cells. J. Biol. Chem. 267, 3000–3007 (1992).
Dohán, O. et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr. Rev. 24, 48–77 (2003).
Raad, H., Eskalli, Z., Corvilain, B., Miot, F. & De Deken, X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2and its maturation factorDUOXA2. Free Radic. Biol. Med. 56, 216–225 (2013).
Cardoso-Weide, L. C. et al. DuOx2 promoter regulation by hormones, transcriptional factors and the coactivator TAZ. Eur. Thyroid J. 4, 6–13 (2015).
Pesce, L. et al. TSH regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Endocrinology 153, 512–521 (2012).
Dupuy, C. et al. Mechanism of hydrogen peroxide formation catalyzed by NADPH oxidase in thyroid plasma membrane. J. Biol. Chem. 266, 3739–3743 (1991).
Song, Y. et al. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J. Clin. Endocrinol. Metab. 95, 375–382 (2010).
Kero, J. et al. Thyrocyte-specific Gq / G11 deficiency impairs thyroid function and prevents goiter development. J. Clin. Invest. 117, 2399–2407 (2007).
Patyra, K. et al. Partial thyrocyte-specific G a s deficiency leads to rapid-onset hypothyroidism, hyperplasia, and papillary thyroid carcinoma – like lesions in mice. FASEB J. 32, 6239–6251 (2018).
Latif, R. et al. New small molecule agonists to the thyrotropin receptor. Thyroid 25, 51–62 (2015).
Luo, Y. et al. A novel role for flotillin-containing lipid rafts in negative-feedback regulation of thyroid-specific gene expression by thyroglobulin. Thyroid 26, 1630–1639 (2016).
Carvalho, D. P. & Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell. Endocrinol. 458, 6–15 (2017).
Huang, H., Shi, Y., Liang, B., Cai, H. & Cai, Q. Iodinated TG in thyroid follicular lumen regulates TTF-1 and PAX8 expression via TSH/TSHR signaling pathway. J. Cell. Biochem. 118, 3444–3451 (2017).
Noguchi, Y. et al. Thyroglobulin (Tg) induces thyroid cell growth in a concentration-specific manner by a mechanism other than thyrotropin / cAMP stimulation. Biochem. Biophys. Res. Commun. 391, 890–894 (2010).
Padron, A. S. et al. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J. Endocrinol. 221, 415–427 (2014).
Schanze, N. et al. 3-iodothyronamine decreases expression of genes involved in iodide metabolism in mouse thyroids and inhibits iodide uptake in PCCL3 thyrocytes. Thyroid 27, 11–22 (2017).
Ferreira, A. C. F. et al. Rapid regulation of thyroid sodium-iodide symporter activity by thyrotrophin and iodine. J. Endocrinol. 184, 69–76 (2005).
Woeber, K. A. Iodine and thyroid disease. Med. Clin. North Am. 75, 169–178 (1991).
Führer, D. et al. Autosomal dominant nonautoimmune hyperthyroidism. Clinical features-diagnosis-therapy. Exp. Clin. Endocrinol. Diabetes 106, 10–15 (1998).
Mai, V. & Burch, H. A. Stepwise approach to the evaluation and treatment of subclinical hyperthyroidism. Endocr. Pract. 18, 772–780 (2012).
Toccafondi, R. et al. Effects of TSH on cAMP levels and thyroid hormone release in human thyroid ‘autonomous’ nodules: relationship with iodothyronine and iodine content in thyroglobulin. Clin. Endocrinol. 17, 537–546 (1982).
Kosugi, S., Hai, N., Okamoto, H., Sugawa, H. & Mori, T. A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur. J. Endocrinol. 143, 471–477 (2000).
Patel, Y. C., Pharoah, P. P. O. D., Hornabrook, R. W. & Hetzel, B. S. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J. Clin. Endocrinol. Metab. 37, 783–789 (1973).
Vagenakis, A. G. et al. Studies of serum triiodothyronine, thyroxine and thyrotropin concentrations in endemic goiter in Greece. J. Clin. Endocrinol. Metab. 37, 485–488 (1973).
Gilbert, J. A. et al. Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in Graves’ disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways. J. Immunol. 176, 5084–5092 (2006).
Targovnik, H. M., Citterio, C. E. & Rivolta, C. M. Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract. Res. Clin. Endocrinol. Metab. 31, 195–212 (2017).
Ricketts, M. H., Simons, M. J., Mercken, L. & Dong, Q. A nonsense mutation causes hereditary goitre in the Afrikander cattle and unmasks alternative splicing of thyroglobulin transcripts. Proc. Natl Acad. Sci. USA 84, 3181–3184 (1987).
Veenboer, G. J. & de Vijlder, J. J. Molecular basis of the thyroglobulin synthesis defect in Dutch goats. Endocrinology 132, 377–381 (1993).
Kim, P. S. et al. A single amino acid change in the acetylcholinesterase-like domain of thyroglobulin causes congenital goiter with hypothyroidism in the cog/cog mouse: a model of human endoplasmic reticulum storage diseases. Proc. Natl Acad. Sci. USA 95, 9909–9913 (1998).
Kim, P. S. et al. A missense mutation G2320R in the thyroglobulin gene causes non-goitrous congenital primary hypothyroidism in the WIC-rdw rat. Mol. Endocrinol. 14, 1944–1953 (2000).
Sato, A. et al. A novel mutation in the thyroglobulin gene that causes goiter and dwarfism in Wistar Hannover GALAS rats. Mutat. Res. 762, 17–23 (2014).
Vigone, M. C., Capalbo, D., Weber, G. & Salerno, M. Mild hypothyroidism in childhood: who, when and how should be treated? J. Endocr. Soc. 2, (1024–1039 (2018).
Nicholas, A. K. et al. Comprehensive screening of eight known causative genes in congenital hypothyroidism with gland-in-situ. J. Clin. Endocrinol. Metab. 101, 4521–4531 (2016).
Peteiro-Gonzalez, D. et al. New insights into thyroglobulin pathophysiology revealed by the study of a family with congenital goiter. J. Clin. Endocrinol. Metab. 95, 3522–3526 (2010).
Kim, P. S. & Arvan, P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of chaperones. Endocr. Rev. 19, 173–202 (1998).
Citterio, C. E. et al. New insights into thyroglobulin gene: molecular analysis of seven novel mutations associated with goiter and hypothyroidism. Mol. Cell. Endocrinol. 365, 277–291 (2013).
Kanou, Y. et al. Thyroglobulin gene mutations producing defective intracellular transport of thyroglobulin are associated with increased thyroidal type 2 iodothyronine deiodinase activity. J. Clin. Endocrinol. Metab. 92, 1451–1457 (2007).
Hishinuma, A. et al. Haplotype analysis reveals founder effects of thyroglobulin gene mutations C1058R and C1977S in Japan. J. Clin. Endocrinol. Metab. 91, 3100–3104 (2006).
Umezu, M., Kagabu, S., Jiang, J. & Sato, E. Evaluation and characterization of congenital hypothyroidism in rdw dwarf rats. Lab. Anim. Sci. 48, 496–501 (1998).
Baryshev, M. et al. Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism. J. Mol. Endocrinol. 32, 903–920 (2004).
Menon, S. et al. Oxidoreductase interactions include a role for ERp72 engagement with mutant thyroglobulin from the rdw/rdw rat dwarf. J. Biol. Chem. 282, 6183–6191 (2007).
Gaide Chevronnay, H. P. et al. A mouse model suggests two mechanisms for thyroid alterations in infantile cystinosis: Decreased thyroglobulin synthesis due to endoplasmic reticulum stress/unfolded protein response and impaired lysosomal processing. Endocrinology 156, 2349–2364 (2015).
Taguchi, A. et al. A symptomatic Fabry disease mouse model generated by inducing globotriaosylceramide synthesis. Biochem. J. 456, 373–383 (2013).
Acknowledgements
The authors acknowledge the support of NIH grant R01 DK40344.
Reviewer information
Nature Reviews Endocrinology thanks X. DeDeken, J. Lado-Abeal and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
C.E.C., H.M.T. and P.A. researched data for the article and made a substantial contribution to discussion of content. C.E.C. and P.A. wrote, reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Metamorphosis
-
An abrupt developmental change in the shape or form of an animal.
- Endostyle
-
A longitudinal, ciliated, grooved organ located on the ventral wall of the pharynx of chordates that has functional equivalence to the vertebrate thyroid gland, among other functions.
- Thyroid hormonogenic coupling reaction
-
A post-translational modification resulting in the physical transfer of a mono-iodotyrosine or di-iodotyrosine donor to a di-iodotyrosine acceptor within a protein.
- Thyroglobulin short unique tail sequence
-
Refers to the final ~32 residues of the thyroglobulin sequence containing the antepenultimate tyrosine residue that can form T3 and is conserved throughout all vertebrates.
- De novo T3 formation
-
Refers to the synthesis of T3 within the thyroglobulin polypeptide backbone that occurs as a result of the coupling of iodotyrosines.
- Thyroglobulin non-hormonogenic tyrosine residues
-
Refers to the tyrosines within thyroglobulin that are not directly involved in thyroid hormone synthesis.
- Haploinsufficiency
-
A condition that arises when there is a complete loss of function of one copy of a gene, and the remaining functional copy of the gene is not adequate to preserve normal function in a diploid organism.
- Monoallelic mutations
-
Changes in the genetic sequence in one of two homologous alleles (paternal or maternal) of a single gene.
Rights and permissions
About this article
Cite this article
Citterio, C.E., Targovnik, H.M. & Arvan, P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 15, 323–338 (2019). https://doi.org/10.1038/s41574-019-0184-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0184-8
This article is cited by
-
GLIS3 expression in the thyroid gland in relation to TSH signaling and regulation of gene expression
Cellular and Molecular Life Sciences (2024)
-
GLIS3 regulates transcription of thyroid hormone biosynthetic genes in coordination with other thyroid transcription factors
Cell & Bioscience (2023)
-
Oxidative stress in Hashimoto’s thyroiditis: possible adjuvant therapies to attenuate deleterious effects
Molecular and Cellular Biochemistry (2023)
-
Toxicological signature for thyroid endocrine disruption of dichlorooctylisothiazolinone in zebrafish larvae
Ecotoxicology (2023)
-
Thyroid function and urinary concentrations of iodine, selenium, and arsenic in vegans, lacto-ovo vegetarians and pescatarians
European Journal of Nutrition (2023)