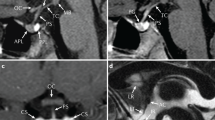
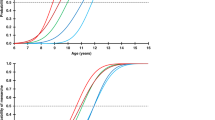
Abstract
In the general population, height is determined by a complex interplay between genetic and environmental factors. Pituitary gigantism is a rare but very important subgroup of patients with excessive height, as it has an identifiable and clinically treatable cause. The disease is caused by chronic growth hormone and insulin-like growth factor 1 secretion from a pituitary somatotrope adenoma that forms before the closure of the epiphyses. If not controlled effectively, this hormonal hypersecretion could lead to extremely elevated final adult height. The past 10 years have seen marked advances in the understanding of pituitary gigantism, including the identification of genetic causes in ~50% of cases, such as mutations in the AIP gene or chromosome Xq26.3 duplications in X-linked acrogigantism syndrome. Pituitary gigantism has a male preponderance, and patients usually have large pituitary adenomas. The large tumour size, together with the young age of patients and frequent resistance to medical therapy, makes the management of pituitary gigantism complex. Early diagnosis and rapid referral for effective therapy appear to improve outcomes in patients with pituitary gigantism; therefore, a high level of clinical suspicion and efficient use of diagnostic resources is key to controlling overgrowth and preventing patients from reaching very elevated final adult heights.
Key points
-
Nearly 50% of patients with pituitary gigantism have a known underlying genetic cause; therefore, these patients should be strongly considered for genetic counselling and screening.
-
Once growth hormone (GH) hypersecretion has been established, efforts should be made to avoid delays in instigating treatment to control levels of GH and insulin-like growth factor 1.
-
A shorter time between diagnosis and the commencement of treatment is associated with a decreased final height in pituitary gigantism.
-
Pituitary gigantism is a disease that predominantly affects males, but males also have a longer delay in time to diagnosis than females, leading to a low proportion of male patients who have disease control by 18 years of age.
-
Somatotropinomas in pituitary gigantism are usually large (macroadenomas) and might be difficult to cure with surgery or medical therapy alone; therefore, multimodal approaches are common in pituitary gigantism.
-
The effect of large tumour size and multiple surgeries and radiotherapy is that patients with pituitary gigantism often have hypopituitarism at long-term follow-up.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jelenkovic, A. et al. Genetic and environmental influences on height from infancy to early adulthood: an individual-based pooled analysis of 45 twin cohorts. Sci. Rep. 6, 28496 (2016).
Jelenkovic, A. et al. Genetic and environmental influences on adult human height across birth cohorts from 1886 to 1994. eLife 5, e20320 (2016).
Berndt, S. I. et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 45, 501–512 (2013).
Macé, A. et al. CNV-association meta-analysis in 191,161 European adults reveals new loci associated with anthropometric traits. Nat. Commun. 8, 744 (2017). This complex analysis shows the importance of CNV as well as mutation on human height.
Zong, X.-N., Li, H., Wu, H.-H. & Zhang, Y.-Q. Socioeconomic development and secular trend in height in China. Econ. Hum. Biol. 19, 258–264 (2015).
Fudvoye, J. & Parent, A.-S. Secular trends in growth. Ann. Endocrinol. (Paris). 78, 88–91 (2017).
Hermanussen, M. et al. Growth variation, final height and secular trend. Proceedings of the 17th Aschauer Soiree, 7th November 2009. HOMO 61, 277–284 (2010).
Judge, T. A. & Cable, D. M. The effect of physical height on workplace success and income: preliminary test of a theoretical model. J. Appl. Physchol. 89, 428–441 (2004).
Deaton, A. S., Hall, W. & School, W. W. Life at the top: the benefits of height. Econ. Hum. Biol. 7, 133–136 (2009).
Lee, J. M. & Howell, J. D. Tall girls: the social shaping of a medical therapy. Arch. Pediatr. Adolesc. Med. 160, 1035–1039 (2006).
Rayner, J.-A., Pyett, P. & Astbury, J. The medicalisation of ‘tall’ girls: a discourse analysis of medical literature on the use of synthetic oestrogen to reduce female height. Soc. Sci. Med. 71, 1076–1083 (2010).
Hannema, S. E. & Sävendahl, L. Clinical practice the evaluation and management of tall stature. Horm. Res. Paediatr. 85, 347–352 (2016).
Davies, J. H. & Cheetham, T. Investigation and management of tall stature. Arch. Dis. Child 99, 772–777 (2014). This article provides a concise and practical overview of the clinical assessment and management of tall stature.
Stalman, S. E., Pons, A., Wit, J. M., Kamp, G. A. & Plötz, F. B. Diagnostic work-up and follow-up in children with tall stature: a simplified algorithm for clinical practice. J. Clin. Res. Pediatr. Endocrinol. 7, 260–267 (2015).
Coutant, R., Donzeau, A., Decrequy, A., Louvigné, M. & Bouhours-Nouet, N. How to investigate a child with excessive growth? Ann. Endocrinol. (Paris). 78, 98–103 (2017).
Baron, J. et al. Short and tall stature: a new paradigm emerges. Nat. Rev. Endocrinol. 11, 735–746 (2015). This paper is an excellent overview of the past findings and future directions of human height research.
Jee, Y. H., Andrade, A. C., Baron, J. & Nilsson, O. Genetics of short stature. Endocrinol. Metab. Clin. North Am. 46, 259–281 (2017).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Committee on Genetics. Health supervision children with Marfan syndrome. Pediatrics 132, e1059–e1072 (2013).
Verge, C. F. & Mowat, D. Overgrowth. Arch. Dis. Child. 95, 458–463 (2010).
Barstow, C. & Rerucha, C. Evaluation of short and tall stature in children. Am. Fam. Physician 92, 43–50 (2015).
Meazza, C., Gertosio, C., Giacchero, R., Pagani, S. & Bozzola, M. Tall stature: a difficult diagnosis? Ital. J. Pediatr. 43, 66 (2017).
Albuquerque, E. V. A., Scalco, R. C. & Jorge, A. A. L. MANAGEMENT OF ENDOCRINE DISEASE: diagnostic and therapeutic approach of tall stature. Eur. J. Endocrinol. 176, R339–R353 (2017).
Stratakis, C. A. A giant? Think of genetics: growth hormone-producing adenomas in the young are almost always the result of genetic defects. Endocrine 50, 272–275 (2015).
Rostomyan, L. et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr. Relat. Cancer 22, 745–757 (2015). This article presents the first international study using standardized data collection to assess the characteristics of pituitary gigantism.
Rostomyan, L., Daly, A. F. & Beckers, A. Pituitary gigantism: causes and clinical characteristics. Ann. Endocrinol. (Paris). 76, 643–649 (2015).
Garnier, E. Enanos y Gigantes (Daniel Cortezo, Barcelona, 1886).
de Herder, W. W. [Giantism. A historical and medical view]. Ned Tijdschr. Geneeskd 148, 2585–2590 (2004).
de Herder, W. W. Acromegaly and gigantism in the medical literature. Case descriptions in the era before and the early years after the initial publication of Pierre Marie (1886). Pituitary 12, 236–244 (2009). This paper provides expert historical background of the medical study of gigantism and acromegaly.
Fritsche, E. & Klebs, E. Ein Beitrag zur Pathologie des Riesenwuchses. Klinische und Pathologisch -Anatomische Untersuchungen (F. C. W. Vogel, 1884).
Marie, P. Sur deux cas d’acromegalie. Hypertrophe singuliere no congénitale des extréméties supérieures, inférieures et cephalique [French]. Révue Méd. Française 6, 297–333 (1886).
Marie, P. & de Souza Leite, J. D. Essays on Acromegaly (The New Sydenham Society, 1891).
de Herder, W. W. A postcard of a giant and his physician (collection de Herder, W. W.). J. Endocrinol. Invest. 28, 392 (2005).
Dana, C. L. Giants and gigantism. Scribner’ Mag. 17, 179–185 (1895).
Hinsdale, G. Acromegaly: an essay to which was awarded the Boylston Prize of Harvard University for the year 1898 (William W. Warren, 1898).
Launois, P. É. & Roy, P. Études Biologiques sur les Géants (Masson et Cie, 1904).
Dufrane, A., Launois, P. E. & Roy, P. Les Relations du Gigantisme et L’acromegalie Expliquees par L’autopsie du Géant Constantin (Masson, Paris, 1903).
Launois, P. É. in Comptes Rendus de l’Association des Anatomistes, Cinquième Session (ed. Nicolas, A.) 51–54 (Institute Anatomique, 1903).
Cushing, H. The Pituitary Body and Its Disorders (J. B. Lippincott Company, 1912).
Beclère, A. Le traitement medical des tumeurs hypophysaires, du gigantisme et de I’acromégalie par la radiothérapie [French]. Arch. d’Electricité Méd. Exp. Clin. 17, 163–180 (1909).
Melmed, S. et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 16, 294–302 (2013).
Katznelson, L. et al. Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99, 3933–3951 (2014).
Petrossians, P. et al. Acromegaly at diagnosis in 3173 patients from the Liège Acromegaly Survey (LAS) database. Endocr. Relat. Cancer 24, 505–518 (2017).
Beckers, A. et al. X-Linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr. Relat. Cancer 22, 353–367 (2015). This article presents a detailed assessment of the unique clinical features of X-LAG syndrome.
Trivellin, G. et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N. Engl. J. Med. 371, 2363–2374 (2014). This publication presents the discovery and description of X-LAG syndrome.
Beckers, A., Aaltonen, L. A., Daly, A. F. & Karhu, A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr. Rev. 34, (239–277 (2013).
Daly, A. F. & Beckers, A. Familial isolated pituitary adenomas (FIPA) and mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrinol. Metab. Clin. North Am. 44, (19–25 (2015).
Martucci, F., Trivellin, G. & Korbonits, M. Familial isolated pituitary adenomas: an emerging clinical entity. J. Endocrinol. Invest. 35, 1003–1014 (2012).
Hannah-Shmouni, F., Trivellin, G. & Stratakis, C. A. Genetics of gigantism and acromegaly. Growth Horm. IGF Res. 30–31, 37–41 (2016).
Daly, A. F. et al. Clinical characterization of familial isolated pituitary adenomas. J. Clin. Endocrinol. Metab. 91, 3316–3323 (2006). This article presents the initial clinical description and definition of FIPAs, the most frequently encountered familial pituitary adenoma presentation.
Daly, A. F. et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J. Clin. Endocrinol. Metab. 95, E373–E383 (2010). This large cohort study establishes the significant differences in disease characteristics and therapeutic responses between acromegaly and gigantism due to AIP mutations versus non-mutated cases.
Hernández-Ramírez, L. C. et al. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J. Clin. Endocrinol. Metab. 100, E1242–E1254 (2015). This paper provides an in-depth analysis of the role of AIP mutations in sporadic and familial pituitary adenoma.
Trivellin, G. & Korbonits, M. AIP and its interacting partners. J. Endocrinol. 210, 137–155 (2011).
Chahal, H. S. et al. Somatostatin analogs modulate AIP in somatotroph adenomas: the role of the ZAC1 pathway. J. Clin. Endocrinol. Metab. 97, 1–10 (2012).
Bolger, G. B. et al. Attenuation of the activity of the cAMP-specific phosphodiesterase PDE4A5 by interaction with the immunophilin XAP2. J. Biol. Chem. 278, 33351–33363 (2003).
Ritvonen, E. et al. Impact of AIP and inhibitory G protein alpha 2 proteins on clinical features of sporadic GH-secreting pituitary adenomas. Eur. J. Endocrinol. 176, 243–252 (2017).
Tuominen, I. et al. AIP inactivation leads to pituitary tumorigenesis through defective Gαi-cAMP signaling. Oncogene 34, 1174–1184 (2015).
de Oliveira, S. K. & Smolenski, A. Phosphodiesterases link the aryl hydrocarbon receptor complex to cyclic nucleotide signaling. Biochem. Pharmacol. 77, 723–733 (2009).
Linnert, M. et al. The FKBP-type domain of the human aryl hydrocarbon receptor-interacting protein reveals an unusual Hsp90 interaction. Biochemistry 52, 2097–2107 (2013).
Formosa, R., Xuereb-Anastasi, A. & Vassallo, J. AIP regulates cAMP signalling and growth hormone secretion in GH3 cells. Endocr. Relat. Cancer 20, 495–505 (2013).
Lecoq, A.-L. et al. AIP mutations impair AhR signaling in pituitary adenoma patients fibroblasts and in GH3 cells. Endocr. Relat. Cancer 23, 433–443 (2016).
Hernández-Ramírez, L. C. et al. Multi-chaperone function modulation and association with cytoskeletal proteins are key features of the function of AIP in the pituitary gland. Oncotarget 9, 9177–9198 (2018).
Hernández-Ramírez, L. C. et al. Rapid proteasomal degradation of mutant proteins is the primary mechanism leading to tumorigenesis in patients with missense AIP mutations. J. Clin. Endocrinol. Metab. 101, 3144–3154 (2016).
Morgan, R. M. L. et al. Structure of the TPR domain of AIP: lack of client protein interaction with the C-terminal α-7 helix of the TPR domain of AIP is sufficient for pituitary adenoma predisposition. PLOS ONE 7, e53339 (2012).
Formosa, R. & Vassallo, J. Aryl hydrocarbon receptor–interacting protein (AIP) N-terminus gene mutations identified in pituitary adenoma patients alter protein stability and function. Horm. Cancer 8, (174–184 (2017).
Cannavo, S. et al. Increased prevalence of acromegaly in a highly polluted area. Eur. J. Endocrinol. 163, 509–513 (2010).
Cannavo, S. et al. Acromegaly is more severe in patients with AHR or AIP gene variants living in highly polluted areas. J. Clin. Endocrinol. Metab. 101, (1872–1879 (2016).
Cannavo, S., Trimarchi, F. & Ferraù, F. Acromegaly, genetic variants of the aryl hydrocarbon receptor pathway and environmental burden. Mol. Cell. Endocrinol. 457, 81–88 (2017).
Lin, B. C. et al. Deletion of the aryl hydrocarbon receptor-associated protein 9 leads to cardiac malformation and embryonic lethality. J. Biol. Chem. 282, 35924–35932 (2007).
Raitila, A. et al. Mice with inactivation of aryl hydrocarbon receptor-interacting protein (Aip) display complete penetrance of pituitary adenomas with aberrant ARNT expression. Am. J. Pathol. 177, (1969–1976 (2010).
Lecoq, A.-L. et al. Mild pituitary phenotype in 3- and 12-month-old Aip-deficient male mice. J. Endocrinol. 231, 59–69 (2016).
Gillam, M. P. et al. Somatotroph-specific aip-deficient mice display pretumorigenic alterations in cell-cycle signaling. J. Endocr. Soc. 1, 78–95 (2017).
Villa, C. et al. Hyperplasia-adenoma sequence in pituitary tumorigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocr. Relat. Cancer 18, 347–356 (2011).
Vierimaa, O. et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312, 1228–1230 (2006). This study describes the discovery of AIP mutations as an inherited cause of pituitary adenomas.
Nagata, Y. et al. Growth hormone-producing pituitary adenomas in childhood and young adulthood: clinical features and outcomes. Pituitary 21, 1–9 (2018).
Naves, L. A. et al. Variable pathological and clinical features of a large Brazilian family harboring a mutation in the aryl hydrocarbon receptor-interacting protein gene. Eur. J. Endocrinol. 157, 383–391 (2007).
Georgitsi, M. et al. Molecular diagnosis of pituitary adenoma predisposition caused by aryl hydrocarbon receptor-interacting protein gene mutations. Proc. Natl Acad. Sci. USA 104, 4101–4105 (2007).
Tichomirowa, M. A. et al. High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. Eur. J. Endocrinol. 165, 509–515 (2011).
Daly, A. F. et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J. Clin. Endocrinol. Metab. 92, 1891–1896 (2007).
Cuny, T. et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. Eur. J. Endocrinol. 168, 533–541 (2013).
Stratakis, C. A. et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin. Genet. 78, 457–463 (2010).
Chahal, H. S. et al. AIP mutation in pituitary adenomas in the 18th century and today. N. Engl. J. Med. 364, 43–50 (2011). This historical and genetic study links present-day patients with acromegaly and gigantism to an AIP founder mutation.
Williams, F. et al. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. J. Clin. Endocrinol. Metab. 99, 1122–1131 (2014).
Igreja, S. et al. Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families. Hum. Mutat. 31, 950–960 (2010).
Leontiou, C. A. et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J. Clin. Endocrinol. Metab. 93, 2390–2401 (2008).
Salvatori, R., Daly, A. F., Quinones-Hinojosa, A., Thiry, A. & Beckers, A. A clinically novel AIP mutation in a patient with a very large, apparently sporadic somatotrope adenoma. Endocrinol. Diabetes Metab. Case Rep. 2014, 140048 (2014).
Jaffrain-Rea, M.-L. et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr. Relat. Cancer 16, 1029–1043 (2009).
Jaffrain-Rea, M.-L. et al. Somatostatin analogues increase AIP expression in somatotropinomas, irrespective of Gsp mutations. Endocr. Relat. Cancer 20, 753–766 (2013).
Kasuki, L. et al. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr. Relat. Cancer 19, L25–L29 (2012).
Kasuki Jomori de Pinho, L. et al. Low aryl hydrocarbon receptor-interacting protein expression is a better marker of invasiveness in somatotropinomas than Ki-67 and p53. Neuroendocrinology 94, 39–48 (2011).
Imran, S. A. et al. Unusual AIP mutation and phenocopy in the family of a young patient with acromegalic gigantism. Endocrinol. Diabetes Metab. Case Rep. https://doi.org/10.1530/EDM-17-0092 (2018).
Salvatori, R. et al. In-frame seven amino-acid duplication in AIP arose over the last 3000 years, disrupts protein interaction and stability and is associated with gigantism. Eur. J. Endocrinol. 177, 257–266 (2017).
Radian, S. et al. Increased population risk of AIP-related acromegaly and gigantism in Ireland. Hum. Mutat. 38, 78–85 (2017).
de Herder, W. W. et al. Acromegalic gigantism, physicians and body snatching. Past or present? Pituitary 15, 312–318 (2012).
Vasilev, V. et al. MANAGEMENT OF ENDOCRINE DISEASE: pituitary ‘incidentaloma’: neuroradiological assessment and differential diagnosis. Eur. J. Endocrinol. 175, R171–R184 (2016).
Ezzat, S. et al. The prevalence of pituitary adenomas: a systematic review. Cancer 101, 613–619 (2004).
Rodd, C. et al. Somatic GPR101 duplication causing X-linked acrogigantism (XLAG) - diagnosis and management. J. Clin. Endocrinol. Metab. 101, 1927–1930 (2016).
Naves, L. A. et al. Aggressive tumor growth and clinical evolution in a patient with X-linked acro-gigantism syndrome. Endocrine 51, 236–244 (2016).
Daly, A. F. et al. Somatic mosaicism underlies X-linked acrogigantism syndrome in sporadic male subjects. Endocr. Relat. Cancer 23, 221–233 (2016). This study describes how X-LAG syndrome can be caused by mosaicism for chromosome Xq26.3 duplications and can be detected in the screening of patient cohorts using digital PCR.
Daly, A. F. et al. GHRH excess and blockade in X-LAG syndrome. Endocr. Relat. Cancer 23, 161–170 (2016).
Trivellin, G., Hernández-Ramírez, L. C., Swan, J. & Stratakis, C. A. An orphan G-protein-coupled receptor causes human gigantism and/or acromegaly: molecular biology and clinical correlations. Best Pract. Res. Clin. Endocrinol. Metab. 32, 125–140 (2018).
Iacovazzo, D. et al. Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol. Commun. 4, 56 (2016).
Gordon, R. J. et al. Childhood acromegaly due to X-linked acrogigantism: long term follow-up. Pituitary 19, 560–564 (2016).
Beckers, A. et al. Paleogenetic study of ancient DNA suggestive of X-linked acrogigantism. Endocr. Relat. Cancer 24, L17–L20 (2017).
Beckers, A., Rostomyan, L., Potorac, I., Beckers, P. & Daly, A. F. X-LAG: how did they grow so tall? Ann. Endocrinol. (Paris) 78, 131–136 (2017).
Trivellin, G. et al. Characterization of GPR101 transcript structure and expression patterns. J. Mol. Endocrinol. 57, 97–111 (2016).
Castinetti, F. et al. GPR101 mutations are not a frequent cause of congenital isolated growth hormone deficiency. Horm. Metab. Res. 48, 389–393 (2016).
Kamenický, P., Bouligand, J. & Chanson, P. Gigantism, acromegaly, and GPR101 mutations. N. Engl. J. Med. 372, 1264–1265 (2015).
Ferraù, F. et al. Analysis of GPR101 and AIP genes mutations in acromegaly: a multicentric study. Endocrine 54, 762–767 (2016).
Daly, A. F., Trivellin, G. & Stratakis, C. A. Gigantism, acromegaly, and GPR101 mutations. N. Engl. J. Med. 372, 1265 (2015).
Trivellin, G. et al. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr. Relat. Cancer 23, 357–365 (2016).
Brandi, M. L. et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 86, 5658–5671 (2001).
Thakker, R. V. et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 97, 2990–3011 (2012).
Beckers, A., Betea, D., Valdes Socin, H. & Stevenaert, A. The treatment of sporadic versus MEN1-related pituitary adenomas. J. Intern. Med. 253, 599–605 (2003).
Daly, A. F. & Beckers, A. in The Pituitary 4th edn (ed. Melmed, S.) 619–630 (Elsevier, 2017).
Trouillas, J. et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 32, 534–543 (2008).
Sergeant, C. et al. Transdifferentiation of neuroendocrine cells: gangliocytoma associated with two pituitary adenomas of different lineage in MEN1. Am. J. Surg. Pathol. 41, 849–853 (2017).
Magri, F. et al. Prevalence of double pituitary adenomas in a surgical series: clinical, histological and genetic features. J. Endocrinol. Invest. 33, 325–331 (2010).
Vergès, B. et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J. Clin. Endocrinol. Metab. 87, 457–465 (2002).
Syro, L. V. et al. Pituitary tumors in patients with MEN1 syndrome. Clinics (Sao Paulo) 67 (Suppl. 1), 43–48 (2012).
Stratakis, C. A. et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J. Clin. Endocrinol. Metab. 85, 4776–4780 (2000).
de Laat, J. M. et al. Long-term natural course of pituitary tumors in patients with MEN1: results from the Dutch MEN1 study group (DMSG). J. Clin. Endocrinol. Metab. 100, 3288–3296 (2015). This long-term cohort study of pituitary disease in MEN1 discusses the challenges of a modern screening programme.
Huang, W. & Molitch, M. E. Management of nonfunctioning pituitary adenomas (NFAs): observation. Pituitary 21, 162–167 (2018).
Albright, F., Butler, A. M., Hampton, A. O. & Smith, P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N. Engl. J. Med. 216, 727–746 (1937).
McCune, D. J. & Bruch, H. Osteodystrophia fibrosa. Am. J. Dis. Child. 54, 806 (1937).
Weinstein, L. S. et al. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N. Engl. J. Med. 325, 1688–1695 (1991). This study demonstrates that MAS is a Gα s -related disease.
Schwindinger, W. F., Francomano, C. A. & Levine, M. A. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc. Natl Acad. Sci. USA 89, 5152–5156 (1992).
Dumitrescu, C. E. & Collins, M. T. McCune-Albright syndrome. Orphanet J. Rare Dis. 3, 12 (2008). This paper presents a comprehensive overview of the clinical presentation and pathology of MAS.
Zacharin, M. et al. Gastrointestinal polyps in McCune Albright syndrome. J. Med. Genet. 48, 458–461 (2011).
Wood, L. D. et al. Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Arch. 470, 391–400 (2017).
Vasilev, V. et al. McCune-Albright syndrome: a detailed pathological and genetic analysis of disease effects in an adult patient. J. Clin. Endocrinol. Metab. 99, E2029–E2038 (2014).
Gaujoux, S. et al. Hepatobiliary and pancreatic neoplasms in patients with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 99, E97–E101 (2014).
Salenave, S., Boyce, A. M., Collins, M. T. & Chanson, P. Acromegaly and McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 99, 1955–1969 (2014).
Akintoye, S. O. et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 87, 5104–5112 (2002).
Vortmeyer, A. O. et al. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 97, 2404–2413 (2012).
Schwindinger, W. F., Francomano, C. A., Levine, M. A. & McKusick, V. A. DNA light on the Tegernsee giant. Lancet 338, 1454–1455 (1991).
Vogl, T. J., Nerlich, A., Dresel, S. H. & Bergman, C. CT of the “Tegernsee Giant”: juvenile gigantism and polyostotic fibrous dysplasia. J. Comput. Assist. Tomogr. 18, 319–322 (1994).
Dötsch, J. et al. Gs alpha mutation at codon 201 in pituitary adenoma causing gigantism in a 6-year-old boy with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 81, 3839–3842 (1996).
Queirolo, S. et al. Gigantism with pituitary macroadenoma: an unusual variant of McCune-Albright syndrome. J. Pediatr. Endocrinol. Metab. 22, 177–179 (2009).
Nakagawa, H. et al. Gigantism associated with McCune-Albright’s syndrome. Horm. Metab. Res. 17, 522–527 (1985).
Schoof, E. et al. Five-year follow-up of a 13-year-old boy with a pituitary adenoma causing gigantism-effect of octreotide therapy. Horm. Res. 61, 184–189 (2004).
Lee, J. S. et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J. Rare Dis. 7 (Suppl. 1), S2 (2012).
Boyce, A. M. et al. Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J. Clin. Endocrinol. Metab. 98, E126–E134 (2013).
Collins, M. T., Singer, F. R. & Eugster, E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J. Rare Dis. 7 (Suppl. 1), S4 (2012).
Belsuzarri, T. B. et al. McCune-Albright syndrome with craniofacial dysplasia: clinical review and surgical management. Surg. Neurol. Int. 7, 165 (2016).
Boyce, A. M. et al. Association of hearing loss and otologic outcomes with fibrous dysplasia. JAMA Otolaryngol. Neck Surg. 144, 102 (2018).
Akintoye, S. O. et al. Pegvisomant for the treatment of gsp-mediated growth hormone excess in patients with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 91, 2960–2966 (2006).
Galland, F. et al. McCune-Albright syndrome and acromegaly: effects of hypothalamopituitary radiotherapy and/or pegvisomant in somatostatin analog-resistant patients. J. Clin. Endocrinol. Metab. 91, 4957–4961 (2006).
Kirschner, L. S. et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat. Genet. 26, 89–92 (2000). This study is about the genetic identification of PRKAR1A as the cause of Carney complex.
Carney, J. A., Gordon, H., Carpenter, P. C., Shenoy, B. V. & Go, V. L. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine 64, 270–283 (1985).
Raff, S. B., Carney, J. A., Krugman, D., Doppman, J. L. & Stratakis, C. A. Prolactin secretion abnormalities in patients with the ‘syndrome of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas’ (Carney complex). J. Pediatr. Endocrinol. Metab. 13, 373–379 (2000).
Stergiopoulos, S. G. & Stratakis, C. A. Human tumors associated with Carney complex and germline PRKAR1A mutations: a protein kinase A disease! FEBS Lett. 546, 59–64 (2003).
Stergiopoulos, S. G., Abu-Asab, M. S., Tsokos, M. & Stratakis, C. A. Pituitary pathology in Carney complex patients. Pituitary 7, 73–82 (2004).
Boikos, S. A. & Stratakis, C. A. Pituitary pathology in patients with Carney Complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary 9, 203–209 (2006).
Pack, S. D. et al. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the ‘complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas’ (Carney complex). J. Clin. Endocrinol. Metab. 85, 3860–3865 (2000).
Pellegata, N. S. et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl Acad. Sci. USA 103, 15558–15563 (2006).
Belar, O., De La Hoz, C., Perez-Nanclares, G., Castano, L. & Gaztambide, S. Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clin. Endocrinol. 76, 719–724 (2011).
Occhi, G. et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLOS Genet. 9, e1003350 (2013).
Molatore, S. et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum. Mutat. 31, E1825–E1835 (2010).
Georgitsi, M. et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J. Clin. Endocrinol. Metab. 92, 3321–3325 (2007).
Georgitsi, M. MEN-4 and other multiple endocrine neoplasias due to cyclin-dependent kinase inhibitors (p27(Kip1) and p18(INK4C)) mutations. Best Pract. Res. Clin. Endocrinol. Metab. 24, 425–437 (2010).
Tichomirowa, M. A. et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr. Relat. Cancer 19, 233–241 (2012).
Sambugaro, S. et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5'UTR region. Endocrine 49, 58–64 (2015).
Kiyokawa, H. et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85, 721–732 (1996).
Fero, M. L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85, 733–744 (1996).
Nakayama, K. et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85, 707–720 (1996).
Xekouki, P. et al. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J. Clin. Endocrinol. Metab. 100, E710–E719 (2015). This paper provides an account of the emerging relationship of paraganglioma and phaeochromocytoma risk genes and pituitary tumours.
O’Toole, S. M., Dénes, J., Robledo, M., Stratakis, C. A. & Korbonits, M. 15 YEARS OF PARAGANGLIOMA: the association of pituitary adenomas and phaeochromocytomas or paragangliomas. Endocr. Relat. Cancer 22, T105–T122 (2015).
Dénes, J. et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J. Clin. Endocrinol. Metab. 100, E531–E541 (2015).
Daly, A. F. et al. Pheochromocytomas and pituitary adenomas in three patients with MAX exon deletions. Endocr. Relat. Cancer 25, L37–L42 (2018).
Tudorancea, A. et al. Von Hippel-Lindau disease and aggressive GH-PRL pituitary adenoma in a young boy. Ann. Endocrinol. (Paris). 73, 37–42 (2012).
Xekouki, P. et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J. Clin. Endocrinol. Metab. 97, E357–E366 (2011).
Cambiaso, P. et al. Growth hormone excess in children with neurofibromatosis type-1 and optic glioma. Am. J. Med. Genet. A 173A, 2353–2358 (2017).
Blanchard, G. et al. Systematic MRI in NF1 children under six years of age for the diagnosis of optic pathway gliomas. Study and outcome of a French cohort. Eur. J. Paediatr. Neurol. 20, 275–281 (2016).
Cnossen, M. H. et al. Endocrinologic disorders and optic pathway gliomas in children with neurofibromatosis type 1. Pediatrics 100, 667–670 (1997).
Listernick, R., Ferner, R. E., Liu, G. T. & Gutmann, D. H. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann. Neurol. 61, 189–198 (2007).
Sani, I. & Albanese, A. Endocrine long-term follow-up of children with neurofibromatosis type 1 and optic pathway glioma. Horm. Res. Paediatr. 87, 179–188 (2017).
Segal, L., Darvish-Zargar, M., Dilenge, M.-E., Ortenberg, J. & Polomeno, R. C. Optic pathway gliomas in patients with neurofibromatosis type 1: follow-up of 44 patients. J. AAPOS 14, 155–158 (2010).
Bruzzi, P., Sani, I. & Albanese, A. Reversible growth hormone excess in two girls with neurofibromatosis type 1 and optic pathway glioma. Horm. Res. Paediatr. 84, 414–422 (2015).
Manski, T. J., Haworth, C. S., Duval-Arnould, B. J. & Rushing, E. J. Optic pathway glioma infiltrating into somatostatinergic pathways in a young boy with gigantism. Case report. J. Neurosurg. 81, 595–600 (1994).
Josefson, J. L., Listernick, R., Charrow, J. & Habiby, R. L. Growth hormone excess in children with optic pathway tumors is a transient phenomenon. Horm. Res. Paediatr. 86, 35–38 (2016).
Melmed, S. et al. Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 94, 1509–1517 (2009).
Katznelson, L. et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly — 2011 update: executive summary. Endocr. Pr. 17 (Suppl. 4), 1–44 (2011).
Ezzat, S. et al. Canadian consensus guidelines for the diagnosis and management of acromegaly. Clin. Invest. Med. 29, 29–39 (2006).
Hindmarsh, P. C., Pringle, P. J., Stanhope, R. & Brook, C. G. The effect of a continuous infusion of a somatostatin analogue (octreotide) for two years on growth hormone secretion and height prediction in tall children. Clin. Endocrinol. (Oxf.) 42, 509–515 (1995).
Hindmarsh, P. C., Pringle, P. J., Di Silvio, L. & Brook, C. G. A preliminary report on the role of somatostatin analogue (SMS 201–995) in the management of children with tall stature. Clin. Endocrinol. (Oxf.) 32, 83–91 (1990).
Goldenberg, N. et al. Treatment of pituitary gigantism with the growth hormone receptor antagonist pegvisomant. J. Clin. Endocrinol. Metab. 93, 2953–2956 (2008).
Daniel, A., d’Emden, M. & Duncan, E. Pituitary gigantism treated successfully with the growth hormone receptor antagonist, pegvisomant. Intern. Med. J. 43, 345–347 (2013).
Creo, A. L. & Lteif, A. N. Pituitary gigantism: a retrospective case series. J. Pediatr. Endocrinol. Metab. 29, 597–602 (2016).
Maheshwari, H. G. et al. Long-acting peptidomimergic control of gigantism caused by pituitary acidophilic stem cell adenoma. J. Clin. Endocrinol. Metab. 85, 3409–3416 (2000).
Müssig, K. et al. Pegvisomant treatment in gigantism caused by a growth hormone-secreting giant pituitary adenoma. Exp. Clin. Endocrinol. Diabetes 115, 198–202 (2007).
Rix, M., Laurberg, P., Hoejberg, A. S. & Brock-Jacobsen, B. Pegvisomant therapy in pituitary gigantism: successful treatment in a 12-year-old girl. Eur. J. Endocrinol. 153, 195–201 (2005).
Mangupli, R. et al. Combined treatment with octreotide LAR and pegvisomant in patients with pituitary gigantism: clinical evaluation and genetic screening. Pituitary 19, 507–514 (2016).
Franck, S. E. et al. A multivariable prediction model for pegvisomant dosing: monotherapy and in combination with long-acting somatostatin analogues. Eur. J. Endocrinol. 176, 421–430 (2017).
Neggers, S. J. & van der Lely, A. J. Combination treatment with somatostatin analogues and pegvisomant in acromegaly. Growth Horm. IGF Res. 21, 129–133 (2011).
Trainer, P. J. et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N. Engl. J. Med. 342, 1171–1177 (2000).
Bernabeu, I. et al. Pegvisomant and cabergoline combination therapy in acromegaly. Pituitary 16, 101–108 (2013).
Personnier, C. et al. Clinical features and treatment of pediatric somatotropinoma: case study of an aggressive tumor due to a new AIP mutation and extensive literature review. Horm. Res. Paediatr. 75, 392–402 (2011).
Daly, A. et al. X-Linked acro-gigantism (X-LAG) syndrome: two new cases with long-term follow-up [abstract]. 4th ENEA Workshop https://orbi.uliege.be/handle/2268/189337 (2015).
Petrossians, P. et al. Gross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogs. Eur. J. Endocrinol. 152, 61–66 (2005).
Saldarriaga, C. et al. Postoperative diabetes insipidus and hyponatremia in children after transsphenoidal surgery for adrenocorticotropin hormone and growth hormone secreting adenomas. J. Pediatr. 195, 169–174 (2018).
Hindmarsh, P. C. Long-term follow-up after bilateral percutaneous epiphysiodesis around the knee to reduce excessive predicted final height. Arch. Dis. Child. 103, 207–208 (2018). This article presents an expert assessment of the challenges of epiphysiodesis and outcomes in tall stature.
Edouard, T. What treatment for a child with tall stature? Ann. Endocrinol. (Paris) 78, 104–105 (2017).
Odink, R. J. et al. Reduction of excessive height in boys by bilateral percutaneous epiphysiodesis around the knee. Eur. J. Pediatr. 165, 50–54 (2006).
Benyi, E. et al. Efficacy and safety of percutaneous epiphysiodesis operation around the knee to reduce adult height in extremely tall adolescent girls and boys. Int. J. Pediatr. Endocrinol. 2010, 740629 (2010).
Goedegebuure, W. J. et al. Long-term follow-up after bilateral percutaneous epiphysiodesis around the knee to reduce excessive predicted final height. Arch. Dis. Child. 103, 219–223 (2018).
Acknowledgements
The authors acknowledge the support of an Actions de Recherche Concertées 2017 grant for the GOLIATHS project from Liège Université, a Fonds d’Investissement pour la Recherche Scientifique grant from the Centre Hospitalier Universitaire de Liège and a grant from the Jabbs Foundation, UK.
Reviewer information
Nature Reviews Endocrinology thanks K. Kovacs, S. Cannavò and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.B. and A.F.D. researched the data for the article, contributed to the discussion of content, wrote the article and reviewed and edited the manuscript before submission. P.P. and J.H. researched data for the article and reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Somatotropic axis
-
The hormonal system, including the hypothalamus, pituitary and peripheral tissues, that controls growth hormone secretion from the anterior pituitary gland and insulin-like growth factor 1 mainly from the liver.
- Somatotrope adenoma
-
A benign tumour of the growth hormone-producing cells of the anterior pituitary gland.
- Epiphyses
-
Proximal and distal end portions of long bones that contain cartilaginous growth plates at which longitudinal bone growth occurs until growth plate closure and epiphyseal fusion with the rest of the bone.
- Extrasellar extension
-
Growth of a pituitary adenoma outside of the borders of the sella turcica.
- Haploblock
-
Also known as haplotype block. A set of neighbouring genetic alleles or markers that tend to be inherited together over generations.
- Pneumatization
-
Development of an air space within a sinus, such as the sphenoid sinus, that gradually takes place over childhood and has an influence on the neurosurgical access to the anterior pituitary gland.
- Transsphenoidal
-
Neurosurgical approach to access the pituitary gland in the sella turcica via the sphenoid sinus.
- Sellar floor
-
The base of the sella turcica in which the pituitary gland lies.
- Transfrontal approach
-
A neurosurgical approach to the pituitary gland via the frontal bone of the skull.
- Tumour debulking
-
Removal of tumour tissue to reduce overall tumour size and related symptoms when full tumour resection cannot be performed.
Rights and permissions
About this article
Cite this article
Beckers, A., Petrossians, P., Hanson, J. et al. The causes and consequences of pituitary gigantism. Nat Rev Endocrinol 14, 705–720 (2018). https://doi.org/10.1038/s41574-018-0114-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0114-1
This article is cited by
-
Consensus guideline for the diagnosis and management of pituitary adenomas in childhood and adolescence: Part 1, general recommendations
Nature Reviews Endocrinology (2024)
-
Genetics of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of the Spinal Ligaments
Current Osteoporosis Reports (2023)
-
The role of the dentist and orthodontist in recognizing oro-facial manifestations of acromegaly: a questionnaire-based study
Pituitary (2022)
-
GWAS reveal a role for the central nervous system in regulating weight and weight change in response to exercise
Scientific Reports (2021)
-
Genetics, clinical features and outcomes of non-syndromic pituitary gigantism: experience of a single center from Sao Paulo, Brazil
Pituitary (2021)