Abstract
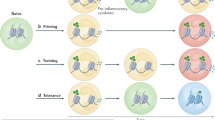
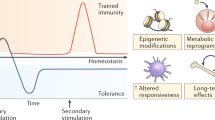
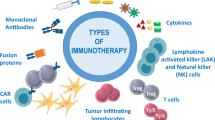
Immunotherapy is revolutionizing the treatment of diseases in which dysregulated immune responses have an important role. However, most of the immunotherapy strategies currently being developed engage the adaptive immune system. In the past decade, both myeloid (monocytes, macrophages and dendritic cells) and lymphoid (natural killer cells and innate lymphoid cells) cell populations of the innate immune system have been shown to display long-term changes in their functional programme through metabolic and epigenetic programming. Such reprogramming causes these cells to be either hyperresponsive or hyporesponsive, resulting in a changed immune response to secondary stimuli. This de facto innate immune memory, which has been termed ‘trained immunity’, provides a powerful ‘targeting framework’ to regulate the delicate balance of immune homeostasis, priming, training and tolerance. In this Opinion article, we set out our vision of how to target innate immune cells and regulate trained immunity to achieve long-term therapeutic benefits in a range of immune-related diseases. These include conditions characterized by excessive trained immunity, such as inflammatory and autoimmune disorders, allergies and cardiovascular disease and conditions driven by defective trained immunity, such as cancer and certain infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lesterhuis, W. J., Haanen, J. B. A. G. & Punt, C. J. A. Cancer immunotherapy – revisited. Nat. Rev. Drug Discov. 10, 591–600 (2011).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Levine, D. B. The Hospital for the Ruptured and Crippled: William Bradley Coley, third Surgeon-in-Chief 1925–1933. HSS J. 4, 1–9 (2008).
Tontonoz, M. Immunotherapy: revolutionizing cancer treatment since 1891. MSKCC.org https://www.mskcc.org/blog/immunotherapy-revolutionizing-cancer-treatment-1891 (2015).
Coley, W. B. II. Contribution to the knowledge of sarcoma. Ann. Surg. 14, 199–220 (1891).
Coley, W. B. The treatment of inoperable sarcoma with the ’mixed toxins of erysipelas and bacillus prodigiosus. J. Am. Med. Assoc. XXXI, 456–465 (1898).
Dinarello, C. A., Simon, A. & van der Meer, J. W. M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012).
Tang, J., Shalabi, A. & Hubbard-Lucey, V. M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 29, 84–91 (2018).
Sharma, P., Wagner, K., Wolchok, J. D. & Allison, J. P. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat. Rev. Cancer 11, 805–812 (2011).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hoos, A. Development of immuno-oncology drugs — from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 15, 235–247 (2016).
Fesnak, A. D., June, C. H. & Levine, B. L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566–581 (2016).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Anguille, S., Smits, E. L., Lion, E., van Tendeloo, V. F. & Berneman, Z. N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 15, e257–e267 (2014).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Old, L. J., Clarke, D. A. & Benacerraf, B. Effect of Bacillus Calmette-Guérin infection on transplanted tumours in the mouse. Nature 184, 291–292 (1959).
Alexandroff, A. B., Jackson, A. M., O’Donnell, M. A. & James, K. BCG immunotherapy of bladder cancer: 20 years on. Lancet 353, 1689–1694 (1999).
Sokal, J. E., Aungst, C. W., Snyderman, M. & Sokal, P. J. Delay in progression of malignant lymphoma after BCG vaccination. N. Engl. J. Med. 291, 1226–1230 (1974).
Morton, D. L. et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann. Surg. 180, 635–643 (1974).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Netea, M. G. & van der Meer, J. W. M. Trained immunity: an ancient way of remembering. Cell Host Microbe 21, 297–300 (2017).
Netea, M. G., Latz, E., Mills, K. H. G. & O’Neill, L. A. J. Innate immune memory: a paradigm shift in understanding host defense. Nat. Immunol. 16, 675–679 (2015).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Buffen, K. et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLOS Pathog. 10, e1004485 (2014).
Medzhitov, R. & Janeway, C. A. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Tosi, M. F. Innate immune responses to infection. J. Allergy Clin. Immunol. 116, 241–249 (2005).
Gasteiger, G. et al. Cellular innate immunity: an old game with new players. J. Innate Immun. 9, 111–125 (2017).
Rock, K. L., Lai, J.-J. & Kono, H. Innate and adaptive immune responses to cell death. Immunol. Rev. 243, 191–205 (2011).
Kurtz, J. Memory in the innate and adaptive immune systems. Microbes Infect. 6, 1410–1417 (2004).
Hong, M. et al. Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun. 6, 6588 (2015).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Bistoni, F. et al. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51, 668–674 (1986).
Bistoni, F. et al. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J. Med. Vet. Mycol. 26, 285–299 (1988).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Cheng, S.-C. et al. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161 (2018).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175 (2018).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 (2018).
Anzai, A. et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J. Exp. Med. 214, 3293–3310 (2017).
Wicks, I. P. & Roberts, A. W. Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol. 12, 37–48 (2016).
Tough, D. F., Tak, P. P., Tarakhovsky, A. & Prinjha, R. K. Epigenetic drug discovery: breaking through the immune barrier. Nat. Rev. Drug Discov. 15, 835–853 (2016).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Chen, F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014).
Barton, E. S. et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447, 326–329 (2007).
Sun, J. C. et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 209, 947–954 (2012).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Kleinnijenhuis, J. et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014).
Eberl, G., Colonna, M., Di Santo, J. P. & McKenzie, A. N. J. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015).
Mattner, J. & Wirtz, S. Friend or foe? The ambiguous role of innate lymphoid cells in cancer development. Trends Immunol. 38, 29–38 (2017).
Halim, T. Y. F. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Martinez-Gonzalez, I. et al. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity 45, 198–208 (2016).
Brown, G. D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003).
US Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00533364 (2010).
US Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00682032 (2018).
US Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00037011 (2013).
Segal, N. H. et al. A phase II efficacy and safety, open-label, multicenter study of imprime PGG injection in combination with cetuximab in patients with stage IV KRAS-mutant colorectal cancer. Clin. Colorectal Cancer 15, 222–227 (2016).
Wang, J. E. et al. Peptidoglycan primes for LPS-induced release of proinflammatory cytokines in whole human blood. Shock 16, 178–182 (2001).
Wray, G. M., Foster, S. J., Hinds, C. J. & Thiemermann, C. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of tumour necrosis factor-alpha, nitric oxide production, shock, and multiple organ injury/dysfunction in the rat. Shock 15, 135–142 (2001).
Girardin, S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Kehraus, S. et al. Novel amino acid derived natural products from the ascidian atriolum robustum: identification and pharmacological characterization of a unique adenosine derivative. J. Med. Chem. 47, 2243–2255 (2004).
Huang, S. Histone methyltransferases, diet nutrients and tumour suppressors. Nat. Rev. Cancer 2, 469–476 (2002).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010).
Arts, R. J. W. et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 17, 2562–2571 (2016).
Arts, R. J. W. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146 (2018).
Carey, B. W., Finley, L. W. S., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
van der Poll, T. & Opal, S. M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 8, 32–43 (2008).
Carson, W. F., Cavassani, K. A., Dou, Y. & Kunkel, S. L. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics 6, 273–283 (2011).
Ishii, M. et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 114, 3244–3254 (2009).
Serafini, P., Borrello, I. & Bronte, V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16, 53–65 (2006).
Zhang, C., Wang, S., Liu, Y. & Yang, C. Epigenetics in myeloid derived suppressor cells: a sheathed sword towards cancer. Oncotarget 7, 57452–57463 (2016).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Arts, R. J. W. et al. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. Oncoimmunology 5, e1229725 (2016).
Arts, R. J. W., Gresnigt, M. S., Joosten, L. A. B. & Netea, M. G. Cellular metabolism of myeloid cells in sepsis. J. Leukoc. Biol. 101, 151–164 (2017).
Park, H., Bourla, A. B., Kastner, D. L., Colbert, R. A. & Siegel, R. M. Lighting the fires within: the cell biology of autoinflammatory diseases. Nat. Rev. Immunol. 12, 570–580 (2012).
Cris¸an, T. O. et al. Uric acid priming in human monocytes is driven by the AKT–PRAS40 autophagy pathway. Proc. Natl Acad. Sci. USA 114, 5485–5490 (2017).
Wendeln, A.-C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Mankad, R. Atherosclerotic vascular disease in the autoimmune rheumatologic patient. Curr. Atheroscler. Rep. 17, 21 (2015).
Bekkering, S., Joosten, L. A. B., van der Meer, J. W. M., Netea, M. G. & Riksen, N. P. The epigenetic memory of monocytes and macrophages as a novel drug target in atherosclerosis. Clin. Ther. 37, 914–923 (2015).
Carney, E. F. Role of podocyte SHP-1 in hyperglycaemic memory. Nat. Rev. Nephrol. 12, 650–650 (2016).
Friedrichs, P. et al. Hyperglycaemic memory affects the neurovascular unit of the retina in a diabetic mouse model. Diabetologia 60, 1354–1358 (2017).
Brasacchio, D. et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58, 1229–1236 (2009).
Häupl, T. et al. Reactivation of rheumatoid arthritis after pregnancy: increased phagocyte and recurring lymphocyte gene activity. Arthritis Rheum. 58, 2981–2992 (2008).
Cejka, D. et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 62, 2294–2302 (2010).
Huber, L. C. et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 56, 1087–1093 (2007).
Rubbert-Roth, A. et al. TNF inhibitors in rheumatoid arthritis and spondyloarthritis: are they the same? Autoimmun. Rev. 17, 24–28 (2018).
Kalden, J. R. & Schulze-Koops, H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat. Rev. Rheumatol. 13, 707–718 (2017).
Lin, Y.-C. et al. Tumor necrosis factor-alpha inhibitors suppress CCL2 chemokine in monocytes via epigenetic modification. Mol. Immunol. 83, 82–91 (2017).
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. Immunometabolic circuits in trained immunity. Semin. Immunol. 28, 425–430 (2016).
Mulder, W. J. M. et al. High-density lipoprotein nanobiologics for precision medicine. Acc. Chem. Res. 51, 127–137 (2018).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015).
Duivenvoorden, R. et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat. Rev. Cardiol. 16, 21–32 (2018).
Ogawa, C., Liu, Y.-J. & Kobayashi, K. S. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr. Bioact. Compd. 7, 180–197 (2011).
Juárez-Verdayes, M. A., Rodríguez-Martínez, S., Cancino-Diaz, M. E. & Cancino-Diaz, J. C. Peptidoglycan and muramyl dipeptide from Staphylococcus aureus induce the expression of VEGF-A in human limbal fibroblasts with the participation of TLR2-NFκB and NOD2-EGFR. Graefes Arch. Clin. Exp. Ophthalmol. 251, 53–62 (2013).
Brown, G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 (2006).
Palma, A. S. et al. Ligands for the β-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J. Biol. Chem. 281, 5771–5779 (2006).
Rickard, D. J. et al. Identification of benzimidazole diamides as selective inhibitors of the nucleotide-binding oligomerization domain 2 (NOD2) signaling pathway. PLOS ONE 8, e69619 (2013).
Tang, C. et al. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 18, 183–197 (2015).
Xie, J. et al. Laminarin-mediated targeting to Dectin-1 enhances antigen-specific immune responses. Biochem. Biophys. Res. Commun. 391, 958–962 (2010).
Dowling, R. J. O., Topisirovic, I., Fonseca, B. D. & Sonenberg, N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim. Biophys. Acta 1804, 433–439 (2010).
Braza, M. S. et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity 49, 819–828 (2018).
Duivenvoorden, R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 5, 3065 (2014).
Youm, Y.-H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Kugelberg, E. Starving inflammation. Nat. Rev. Immunol. 15, 199–199 (2015).
Shao, B.-Z., Xu, Z.-Q., Han, B.-Z., Su, D.-F. & Liu, C. NLRP3 inflammasome and its inhibitors: a review. Front. Pharmacol. 6, 262 (2015).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Tough, D. F., Lewis, H. D., Rioja, I., Lindon, M. J. & Prinjha, R. K. Epigenetic pathway targets for the treatment of disease: accelerating progress in the development of pharmacological tools: IUPHAR review 11. Br. J. Pharmacol. 171, 4981–5010 (2014).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Hamilton, J. A., Cook, A. D. & Tak, P. P. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Discov. 16, 53–70 (2016).
Chakraborty, C., Sharma, A. R., Sharma, G., Doss, C. G. P. & Lee, S.-S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids 8, 132–143 (2017).
Ortved, K. F., Austin, B. S., Scimeca, M. S. & Nixon, A. J. RNA interference mediated interleukin-1 β silencing in inflamed chondrocytes decreases target and downstream catabolic responses. Arthritis 2016, 3484961 (2016).
Hara, K. et al. Interferon-tau attenuates uptake of nanoparticles and secretion of interleukin-1β in macrophages. PLOS ONE 9, e113974 (2014).
Dvir, T., Timko, B. P., Kohane, D. S. & Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 6, 13–22 (2011).
Metselaar, J. M. & Storm, G. Liposomes in the treatment of inflammatory disorders. Expert Opin. Drug Deliv. 2, 465–476 (2005).
Moghimi, S. M., Hunter, A. C. & Murray, J. C. Nanomedicine: current status and future prospects. FASEB J. 19, 311–330 (2005).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
Lammers, T., Kiessling, F., Hennink, W. E. & Storm, G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release 161, 175–187 (2012).
Tang, J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 1, e1400223 (2015).
Tang, J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016).
Lameijer, M. et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2, 279–292 (2018).
Chen, W. et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 24, 1689–1699 (2010).
Perez-Medina, C. et al. PET imaging of tumor-associated macrophages with 89Zr-labeled high-density lipoprotein nanoparticles. J. Nucl. Med. 56, 1272–1277 (2015).
Lobatto, M. E., Fuster, V., Fayad, Z. A. & Mulder, W. J. M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10, 835–852 (2011).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Zanganeh, S. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 11, 986–994 (2016).
Syn, N. L., Teng, M. W. L., Mok, T. S. K. & Soo, R. A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 18, e731–e741 (2017).
Robert, C. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117 (2014).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Goswami, S., Aparicio, A. & Subudhi, S. K. Immune checkpoint therapies in prostate cancer. Cancer J. 22, 117–120 (2016).
Loveridge, C. J. et al. Increased T cell infiltration elicited by Erk5 deletion in a Pten -deficient mouse model of prostate carcinogenesis. Cancer Res. 77, 3158–3168 (2017).
Hamanishi, J., Mandai, M. & Konishi, I. Immune checkpoint inhibition in ovarian cancer. Int. Immunol. 28, 339–348 (2016).
Krieg, C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 24, 144–153 (2018).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-019-0127-6 (2019).
Leentjens, J. et al. Reversal of immunoparalysis in humans in vivo. Am. J. Respir. Crit. Care Med. 186, 838–845 (2012).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Mulder, W. J. M. et al. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 42, 904–914 (2009).
Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F. & Farokhzad, O. C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41, 2971–3010 (2012).
Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005).
van Schooneveld, M. M. et al. Imaging and quantifying the morphology of an organic-inorganic nanoparticle at the sub-nanometre level. Nat. Nanotechnol. 5, 538–544 (2010).
Mieszawska, A. J., Mulder, W. J. M., Fayad, Z. A. & Cormode, D. P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 10, 831–847 (2013).
Zhao, Y. et al. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 7, 11221 (2016).
Pérez-Medina, C. et al. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc. Imaging 9, 950–961 (2016).
Lührs, H. et al. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 37, 458–466 (2002).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Borriello, F. et al. GM-CSF and IL-3 modulate human monocyte TNF-α production and renewal in in vitro models of trained immunity. Front. Immunol. 7, 680 (2016).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R01 CA220234, R01 HL144072, P01 HL131478, and Netherlands Organization for Scientific Research (NWO) grant ZonMW Vici 91818622 (all to W.J.M.M.), as well as NIH grants R01 HL143814 and P01HL131478 (both to Z.A.F.). J.O. is supported by R01 AI139623, as well as SAF2013-48834-R and SAF2016-80031-R grants from the Spanish Government. L.A.B.J. is supported by a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (HINT, P_37_762). M.G.N. is supported by a European Research Council (ERC) Consolidator Grant (#310372) and an NWO Spinoza Prize. The authors thank K. Joyes for editing the manuscript.
Author information
Authors and Affiliations
Contributions
W.J.M.M. and M.G.N. wrote the manuscript. All authors researched data for the article, provided substantial contribution to discussion of content and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they are scientific founders of Trained Therapeutics Discovery.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mulder, W.J.M., Ochando, J., Joosten, L.A.B. et al. Therapeutic targeting of trained immunity. Nat Rev Drug Discov 18, 553–566 (2019). https://doi.org/10.1038/s41573-019-0025-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0025-4
This article is cited by
-
Vaccination with a combination of STING agonist-loaded lipid nanoparticles and CpG-ODNs protects against lung metastasis via the induction of CD11bhighCD27low memory-like NK cells
Experimental Hematology & Oncology (2024)
-
Current challenges and therapeutic advances of CAR-T cell therapy for solid tumors
Cancer Cell International (2024)
-
Cortical microinfarcts potentiate recurrent ischemic injury through NLRP3-dependent trained immunity
Cell Death & Disease (2024)
-
Trained innate immunity modulates osteoblast and osteoclast differentiation
Stem Cell Reviews and Reports (2024)
-
Attenuated Dengue virus PV001-DV induces oncolytic tumor cell death and potent immune responses
Journal of Translational Medicine (2023)