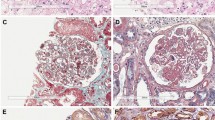
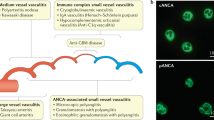
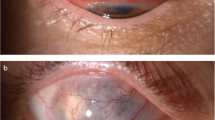
Abstract
Cryoglobulinaemia refers to the serum presence of cryoglobulins, which are defined as immunoglobulins that precipitate at temperatures <37 °C. Type I cryoglobulinaemia consists of only one isotype or subclass of monoclonal immunoglobulin, whereas type II and type III are classified as mixed cryoglobulinaemia because they include immunoglobulin G (IgG) and IgM. Many lymphoproliferative, infectious and autoimmune disorders have been associated with mixed cryoglobulinaemia; however, hepatitis C virus (HCV) is the aetiologic agent in most patients. The underlying mechanism of the disorder is B cell lymphoproliferation and autoantibody production. Mixed cryoglobulinaemia can cause systemic vasculitis, with manifestations ranging from purpura, arthralgia and weakness to more serious lesions with skin ulcers, neurological and renal involvement. This Primer focuses on mixed cryoglobulinaemia, which has a variable course and a prognosis that is primarily influenced by vasculitis-associated multiorgan damage. In addition, the underlying associated disease in itself may cause considerable mortality and morbidity. Treatment of cryoglobulinaemic vasculitis should be modulated according to the underlying associated disease and the severity of organ involvement and relies on antiviral treatment (for HCV infection), immunosuppression and immunotherapy, particularly anti-CD20 B cell depletion therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brouet, J. C., Clauvel, J. P., Danon, F., Klein, M. & Seligmann, M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am. J. Med. 57, 775–788 (1974).
Ramos-Casals, M., Stone, J. H., Cid, M. C. & Bosch, X. The cryoglobulinaemias. Lancet 379, 348–360 (2012).
Ferri, C. et al. Association between hepatitis C virus and mixed cryoglobulinemia [see comment]. Clin. Exp. Rheumatol. 9, 621–624 (1991).
Ferri, C. et al. B cells and mixed cryoglobulinemia. Autoimmun. Rev. 7, 114–120 (2007).
Jennette, J. C. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 37, 187–192 (1994).
Jennette, J. et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Monti, G. et al. Prevalence of mixed cryoglobulinaemia syndrome and circulating cryoglobulins in a population-based survey: the Origgio study. Autoimmun. Rev. 13, 609–614 (2014).
Cacoub, P. et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 42, 2204–2212 (1999).
Ramos-Casals, M., Trejo, O., García-Carrasco, M., Cervera, R. & Font, J. Mixed cryoglobulinemia: new concepts. Lupus 9, 83–91 (2000).
Ferri, C. et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 34, 1606–1610 (1991). This study pioneers the notion that viral agents (including HCV) have a role in the pathogenesis of mixed cryoglobulinaemia patients.
Casato, M. et al. Cryoglobulinaemia and hepatitis C virus. Lancet 337, 1047–1048 (1991).
Disdier, P., Harlé, J. R. & Weiller, P. J. Cryoglobulinaemia and hepatitis C infection. Lancet 338, 1151–1152 (1991).
Arribas, J. R. et al. Association between hepatitis C virus and mixed cryoglobulinemia. Rev. Infect. Dis. 13, 770–771 (1991).
Mohd Hanafiah, K., Groeger, J., Flaxman, A. D. & Wiersma, S. T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57, 1333–1342 (2013).
Kayali, Z., Buckwold, V. E., Zimmerman, B. & Schmidt, W. N. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology 36, 978–985 (2002).
Lunel, F. et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology 106, 1291–1300 (1994).
Adinolfi, L. E. et al. Epidemiology, clinical spectrum and prognostic value of mixed cryoglobulinaemia in hepatitis C virus patients: a prospective study. Ital. J. Gastroenterol. 28, 1–9 (1996).
Saadoun, D. et al. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology 43, 1337–1345 (2006).
Terrier, B. et al. Non HCV-related infectious cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey and systematic review of the literature. J. Autoimmun. 65, 74–81 (2015). This article presents a nationwide survey that included patients with HCV-negative cryoglobulinaemia vasculitis, which describes the presentation, therapeutic management and outcome of patients with non-HCV infectious cryoglobulinaemia vasculitis.
Ferri, C. et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin. Arthritis Rheum. 33, 355–374 (2004).
Scotto, G. et al. Cryoglobulinemia in subjects with HCV infection alone, HIV infection and HCV/HIV coinfection. J. Infect. 52, 294–299 (2006).
García-Carrasco, M. et al. Cryoglobulinemia in systemic lupus erythematosus: prevalence and clinical characteristics in a series of 122 patients. Semin. Arthritis Rheum. 30, 366–373 (2001).
Tzioufas, A. G. et al. Cryoglobulinemia in autoimmune rheumatic diseases. Evidence of circulating monoclonal cryoglobulins in patients with primary Sjögren’s syndrome. Arthritis Rheum. 29, 1098–1104 (1986). This study suggests that primary Sjögren syndrome expresses, in addition to polyclonal B cell hyper-reactivity, a monoclonal process in the absence of lymphoid neoplasia; it showed that the extraglandular manifestations of the syndrome may be due to an immune-complex-mediated pathology.
Brito-Zerón, P. et al. Sjögren syndrome. Nat. Rev. Dis. Primers 2, 16047 (2016).
Monti, G. et al. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch. Intern. Med. 165, 101–105 (2005).
Saadoun, D., Landau, D. A., Calabrese, L. H. & Cacoub, P. P. Hepatitis C-associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology (Oxford) 46, 1234–1242 (2007).
Besson, C. et al. Characteristics and outcome of diffuse large B cell lymphoma in hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des Lymphomes de l’Adulte programs. J. Clin. Oncol. 24, 953–960 (2006).
Néel, A. et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am. J. Hematol. 89, 156–161 (2014).
Roccatello, D. et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am. J. Kidney Dis. 49, 69–82 (2007). In the largest available cohort of cryoglobulin-associated biopsy-proven glomerulonephritis, this study confirms the close association between mixed cryoglobulinaemia, HCV infection, type II cryoglobulin and typical histological features.
Fornasieri, A. et al. Glomerulonephritis induced by human IgMK-IgG cryoglobulins in mice. Lab. Invest. 69, 531–540 (1993).
Grey, H. M. & Kohler, P. F. Cryoimmunoglobulins. Semin. Hematol. 10, 87–112 (1973).
Ferri, C. et al. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br. J. Haematol. 88, 392–394 (1994). This study provides the first insight about the crosslink between haematological malignancies, mainly B cell lymphomas, and HCV infection.
Pileri, P. et al. Binding of hepatitis C virus to CD81. Science 282, 938–941 (1998).
Morsica, G. et al. Replication of hepatitis C virus in B lymphocytes (CD19+). Blood 94, 1138–1139 (1999).
Ito, M. et al. Enhanced expression of lymphomagenesis-related genes in peripheral blood B cells of chronic hepatitis C patients. Clin. Immunol. 135, 459–465 (2010).
Caussin-Schwemling, C., Schmitt, C. & Stoll-Keller, F. Study of the infection of human blood derived monocyte/macrophages with hepatitis C virus in vitro. J. Med. Virol. 65, 14–22 (2001).
Navas, M.-C. et al. Dendritic cell susceptibility to hepatitis C virus genotype 1 infection. J. Med. Virol. 67, 152–161 (2002).
Agnello, V., Chung, R. T. & Kaplan, L. M. A role for hepatitis C virus infection in type II cryoglobulinemia. N. Engl. J. Med. 327, 1490–1495 (1992).
Sansonno, D. et al. Detection and distribution of hepatitis C virus-related proteins in lymph nodes of patients with type II mixed cryoglobulinemia and neoplastic or non-neoplastic lymphoproliferation. Blood 88, 4638–4645 (1996).
De Vita, S. et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood 86, 1887–1892 (1995).
Giordano, T. P. et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 297, 2010–2017 (2007).
Hermine, O. et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N. Engl. J. Med. 347, 89–94 (2002).
Charles, E. D. & Dustin, L. B. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 76, 818–824 (2009).
Charles, E. D. et al. Clonal expansion of immunoglobulin M+CD27+B cells in HCV-associated mixed cryoglobulinemia. Blood 111, 1344–1356 (2008).
De Vita, S. et al. Gastric mucosa as an additional extrahepatic localization of hepatitis C virus: viral detection in gastric low-grade lymphoma associated with autoimmune disease and in chronic gastritis. Hepatology 31, 182–189 (2000).
Isnardi, I. et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood 115, 5026–5036 (2010).
Ivanovski, M. et al. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood 91, 2433–2442 (1998).
Flint, M. et al. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J. Virol. 73, 6782–6790 (1999).
Yagnik, A. T. et al. A model for the hepatitis C virus envelope glycoprotein E2. Proteins 40, 355–366 (2000).
Ferri, C., Pileri, S. & Zignego, A. L. in Infectious Causes of Cancer (ed. Goedert, J. J.) 349–368 (2000).
Roccatello, D. et al. Impaired hepatosplenic elimination of circulating cryoglobulins in patients with essential mixed cryoglobulinaemia and hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 110, 9–14 (1997).
Ferri, C. et al. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig. Liver Dis. 39 (Suppl. 1), S13–S21 (2007).
Ferri, C. et al. Hepatitis C virus syndrome: a constellation of organ- and non-organ specific autoimmune disorders, B cell non-Hodgkin’s lymphoma, and cancer. World J. Hepatol. 7, 327–343 (2015).
Roccatello, D. et al. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol. Dial. Transplant. 19, 3054–3061 (2004). This study presents the first available evidence of the safety and efficacy of anti-CD20 monoclonal antibody in cryoglobulinaemic glomerulonephritis.
Roccatello, D., Giachino, O., Menegatti, E. & Baldovino, S. Relationship between cryoglobulinemia-associated nephritis and HCV infection. Expert Rev. Clin. Immunol. 4, 515–524 (2008).
Sansonno, D. & Dammacco, F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect. Dis. 5, 227–236 (2005).
Roccasecca, R. et al. Binding of the hepatitis C virus E2 glycoprotein to CD81 is strain specific and is modulated by a complex interplay between hypervariable regions 1 and 2. J. Virol. 77, 1856–1867 (2003).
Petracca, R. et al. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J. Virol. 74, 4824–4830 (2000).
Rosa, D. et al. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc. Natl Acad. Sci. USA 102, 18544–18549 (2005).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
De Sanjose, S. et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin. Gastroenterol. Hepatol. 6, 451–458 (2008).
Roccatello, D. et al. Role of monocytes in cryoglobulinemia-associated nephritis. Kidney Int. 43, 1150–1155 (1993).
D’Amico, G., Colasanti, G., Ferrario, F. & Sinico, R. A. Renal involvement in essential mixed cryoglobulinemia. Kidney Int. 35, 1004–1014 (1989).
Guo, S. et al. Macrophages are essential contributors to kidney injury in murine cryoglobulinemic membranoproliferative glomerulonephritis. Kidney Int. 80, 946–958 (2011).
Menegatti, E. et al. Immunogenetics of complement in mixed cryoglobulinaemia. Clin. Exp. Rheumatol. 34 (3 Suppl. 97), S12–S15 (2016).
Ferri, C., Zignego, A. L. & Pileri, S. A. Cryoglobulins. J. Clin. Pathol. 55, 4–13 (2002).
De Vita, S. et al. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann. Rheum. Dis. 70, 1183–1190 (2011).
Pietrogrande, M. et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun. Rev. 10, 444–454 (2011).
Stone, M. J. & Bogen, S. A. Evidence-based focused review of management of hyperviscosity syndrome. Blood 119, 2205–2208 (2012).
Terrier, B. et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore) 92, 61–68 (2013).
Trejo, O. et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore) 80, 252–262 (2001).
Quartuccio, L. et al. Validation of the classification criteria for cryoglobulinaemic vasculitis. Rheumatology 53, 2209–2213 (2014).
McLeod, B. C. & Sassetti, R. J. ‘Hypocryoglobulins’. enhanced cryoprecipitation from hypotonic serum in patients with vasculitis. Arch. Intern. Med. 144, 1381–1385 (1984).
Ferri, C. et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun. Rev. 15, 1145–1160 (2016). A multidisciplinary network of experts, the International Study Group of Extrahepatic Manifestations Related to Hepatitis C Virus Infection (ISG-EHCV), was organized with the intention to formulate diagnostic guidelines for the work-up of possible HCV-related extrahepatic manifestations.
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann. Rheum. Dis. 76, 9–16 (2017).
Retamozo, S., Brito-Zerón, P., Bosch, X., Stone, J. H. & Ramos-Casals, M. Cryoglobulinemic disease. Oncology (Williston Park) 27, 1098–1105, 1110–1116 (2013).
Landau, D.-A. et al. Causes and predictive factors of mortality in a cohort of patients with hepatitis C virus-related cryoglobulinemic vasculitis treated with antiviral therapy. J. Rheumatol. 37, 615–621 (2010).
Terrier, B. et al. Prognostic factors in patients with hepatitis C virus infection and systemic vasculitis. Arthritis Rheum. 63, 1748–1757 (2011).
Saadoun, D. et al. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch. Intern. Med. 166, 2101–2108 (2006).
Saadoun, D. et al. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology 153, 49–52.e5 (2017). This article presents an open-label, prospective, multicentre study of the effectiveness and tolerance of an all-oral, interferon-free and ribavirin-free regimen of sofosbuvir plus daclatasvir in patients with HCV-associated cryoglobulinaemia vasculitis.
Tarantino, A. et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 47, 618–623 (1995).
Dammacco, F. et al. Natural interferon-alpha versus its combination with 6-methyl-prednisolone in the therapy of type II mixed cryoglobulinemia: a long-term, randomized, controlled study. Blood 84, 3336–3343 (1994).
Fabrizi, F. et al. Interferon therapy for HCV-associated glomerulonephritis: meta-analysis of controlled trials. Int. J. Artif. Organs 30, 212–219 (2007).
Gobbi, M. & Scudeletti, M. Deflazacort in the treatment of haematologic disorders. Eur. J. Clin. Pharmacol. 45 (Suppl. 1), S25–S28 (1993).
Roccatello, D. et al. Improved (4 plus 2) Rituximab protocol for severe cases of mixed cryoglobulinemia: a 6-year observational study. Am. J. Nephrol. 43, 251–260 (2016).
Quartuccio, L. et al. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford) 45, 842–846 (2006).
Visentini, M. et al. Efficacy of low-dose rituximab for the treatment of mixed cryoglobulinemia vasculitis: phase II clinical trial and systematic review. Autoimmun. Rev. 14, 889–896 (2015).
De Vita, S., Quartuccio, L. & Fabris, M. Rituximab in mixed cryoglobulinemia: increased experience and perspectives. Dig. Liver Dis. 39, 122–128 (2007).
Visentini, M. et al. A phase II, single-arm multicenter study of low-dose rituximab for refractory mixed cryoglobulinemia secondary to hepatitis C virus infection. Autoimmun. Rev. 10, 714–719 (2011).
Dammacco, F. et al. Pegylated interferon-, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood 116, 343–353 (2010).
Ferri, C. et al. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun. Rev. 11, 48–55 (2011).
Petrarca, A. et al. Safety and efficacy of rituximab in patients with hepatitis C virus-related mixed cryoglobulinemia and severe liver disease. Blood 116, 335–342 (2010).
Pellicelli, A. M. & Zoli, V. Role of ribavirin in hepatitis flare in HCV-infected patients with B cell non Hodgkin’s lymphoma treated with rituximab-containing regimens. Dig. Liver Dis. 43, 501–502 (2011).
Matsue, K. et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B cell lymphoma. Cancer 116, 4769–4776 (2010).
Cavallo, R. et al. Rituximab in cryoglobulinemic peripheral neuropathy. J. Neurol. 256, 1076–1082 (2009).
Mazzi, G. et al. Plasma-exchange in chronic peripheral neurological disorders. Int. J. Artif. Organs 22, 40–46 (1999).
Sinico, R. A. et al. Plasma exchange in the treatment of essential mixed cryoglobulinemia nephropathy. Long-term follow up. Int. J. Artif. Organs 8 (Suppl. 2), 15–18 (1985).
L’Abbate, A. et al. Long term effects of cryoapheresis and cytostatic treatment in essential mixed cryoglobulinemia. Int. J. Artif. Organs 8 (Suppl. 2), 19–22 (1985).
Li, X. et al. Prognostic analysis of acute exacerbations of hepatitis-B after chemotherapy in combination with rituximab in 19 patients with lymphoma. Leuk. Lymphoma 51, 1678–1685 (2010).
Marignani, M. et al. HCV-positive status and hepatitis flares in patients with B cell non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Dig. Liver Dis. 43, 139–142 (2011).
Ramos-Casals, M. et al. Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J. Hepatol. 66, 1282–1299 (2017).
Sidana, S. et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am. J. Hematol. 92, 668–673 (2017).
Harel, S. et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br. J. Haematol. 168, 671–678 (2015).
Retamozo, S., Brito-Zerón, P., Quartuccio, L., De Vita, S. & Ramos-Casals, M. Introducing treat-to-target strategies of autoimmune extrahepatic manifestations of chronic hepatitis C virus infection. Expert Rev. Clin. Pharmacol. 10, 1085–1101 (2017).
Ramos-Casals, M. et al. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin. Arthritis Rheum. 36, 189–196 (2006).
Quartuccio, L. et al. Retreatment regimen of rituximab monotherapy given at the relapse of severe HCV-related cryoglobulinemic vasculitis: long-term follow up data of a randomized controlled multicentre study. J. Autoimmun. 63, 88–93 (2015).
Roccatello, D. et al. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia. Clin. Rev. Allergy Immunol. 34, 111–117 (2008).
Roccatello, D. et al. The challenge of treating hepatitis C virus-associated cryoglobulinemic vasculitis in the era of anti-CD20 monoclonal antibodies and direct antiviral agents. Oncotarget 8, 41764–41777 (2017). This paper presents a comparative evaluation between two different therapeutic approaches to HCV-associated cryoglobulinaemia vasculitis.
Galli, M. et al. HCV-unrelated cryoglobulinaemic vasculitis: the results of a prospective observational study by the Italian Group for the Study of Cryoglobulinaemias (GISC). Clin. Exp. Rheumatol. 35 (Suppl. 1), 67–76 (2017).
Mazzaro, C. et al. Hepatitis B virus related cryoglobulinemic vasculitis: a multicentre open label study from the Gruppo Italiano di Studio delle Crioglobulinemie – GISC. Dig. Liver Dis. 48, 780–784 (2016).
Retamozo, S. et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary Sjögren syndrome: analysis of 515 patients. Rheumatology (Oxford) 55, 1443–1451 (2016).
Terrier, B. et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res. (Hoboken) 62, 1787–1795 (2010).
Terrier, B. et al. Predictors of early relapse in patients with non-infectious mixed cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey. Autoimmun. Rev. 13, 630–634 (2014).
Quartuccio, L. et al. Performance of the preliminary classification criteria for cryoglobulinaemic vasculitis and clinical manifestations in hepatitis C virus-unrelated cryoglobulinaemic vasculitis. Clin. Exp. Rheumatol. 30, S48–S52 (2012).
Younossi, Z., Park, H., Henry, L., Adeyemi, A. & Stepanova, M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 150, 1599–1608 (2016).
Scarpato, S. et al. Pain management in cryoglobulinaemic syndrome. Best Pract. Res. Clin. Rheumatol. 29, 77–89 (2015).
Seaman, K., Paterson, B. L., Vallis, M., Hirsch, G. & Peltekian, K. M. Future directions for investigation of fatigue in chronic hepatitis C viral infection. Chronic Illn. 5, 115–128 (2009).
Monaco, S. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders: advances in 2015. World J. Gastroenterol. 21, 11974 (2015).
Younossi, Z. M. et al. Association of work productivity with clinical and patient-reported factors in patients infected with hepatitis C virus. J. Viral Hepat. 23, 623–630 (2016).
Meltzer, M., Franklin, E. C., Elias, K., McCluskey, R. T. & Cooper, N. Cryoglobulinemia — a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am. J. Med. 40, 837–856 (1966).
Pascual, M., Perrin, L., Giostra, E. & Schifferli, J. A. Hepatitis C virus in patients with cryoglobulinemia type II. J. Infect. Dis. 162, 569–570 (1990).
Ferri, C. et al. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood 82, 3701–3704 (1993).
Landau, D. A. et al. Correlation of clinical and virologic responses to antiviral treatment and regulatory T cell evolution in patients with hepatitis C virus-induced mixed cryoglobulinemia vasculitis. Arthritis Rheum. 58, 2897–2907 (2008).
Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum 64, 835–42 (2012).
De Vita S, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum 64, 843–53 (2012).
Roccatello, D. et al. The ‘4 plus 2’ rituximab protocol makes maintenance treatment unneeded in patients with refractory ANCA-associated vasculitis: a 10 years observation study. Oncotarget 8, 52072–52077 (2017).
Joseph, A. M. Treatment of rheumatoid arthritis in patients with concomitant chronic hepatitis C infection. Ther. Adv. Musculoskelet. Dis. 4, 35–40 (2012).
Cohen, C. et al. Efficacy of tocilizumab in rituximab-refractory cryoglobulinemia vasculitis. Ann. Rheum. Dis. 71, 628–629 (2012).
Lake-Bakaar, G., Jacobson, I. & Talal, A. B cell activating factor (BAFF) in the natural history of chronic hepatitis C virus liver disease and mixed cryoglobulinaemia. Clin. Exp. Immunol. 170, 231–237 (2012).
Saadoun, D. et al. Regulatory T cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Zignego, A. L. et al. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun. Rev. 16, 523–541 (2017).
Ferri, C. et al. Effect of alpha-interferon on hepatitis C virus chronic infection in mixed cryoglobulinemia patients. Infection 21, 93–97 (1993).
Mazzaro, C. et al. Efficacy and safety of peginterferon alfa-2b plus ribavirin for HCV-positive mixed cryoglobulinemia: a multicentre open-label study. Clin. Exp. Rheumatol. 29, 933–941 (2011).
Gragnani, L. et al. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: a prospective, controlled, open-label, cohort study. Hepatology 61, 1145–1153 (2015).
La Civita, L. et al. Exacerbation of peripheral neuropathy during alpha-interferon therapy in a patient with mixed cryoglobulinemia and hepatitis B virus infection. J. Rheumatol. 23, 1641–1643 (1996).
Banerjee, D. & Reddy, K. R. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment. Pharmacol. Ther. 43, 674–696 (2016).
Saadoun, D. et al. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann. Rheum. Dis. 75, 1777–1782 (2016).
Gragnani, L. et al. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology 64, 1473–1482 (2016). Interferon-free, guideline-tailored therapy with direct-acting antivirals is highly effective and safe for HCV-associated patients with mixed cryoglobulinaemia.
Bonacci, M. et al. Virologic, clinical, and immune response outcomes of patients with hepatitis C virus–associated cryoglobulinemia treated with direct-acting antivirals. Clin. Gastroenterol. Hepatol. 15, 575–583.e1 (2017).
Lauletta, G., Russi, S., Pavone, F., Vacca, A. & Dammacco, F. Direct-acting antiviral agents in the therapy of hepatitis C virus-related mixed cryoglobulinaemia: a single-centre experience. Arthritis Res. Ther. 19, 74 (2017).
Emery, J. S. et al. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am. J. Gastroenterol. 112, 1298–1308 (2017).
Kondili, L. A. & Vella, S. PITER Collaborating Group. PITER: an ongoing nationwide study on the real-life impact of direct acting antiviral based treatment for chronic hepatitis C in Italy. Dig. Liver Dis. 47, 741–743 (2015).
Cacoub, P., Desbois, A. C., Isnard-Bagnis, C., Rocatello, D. & Ferri, C. Hepatitis C virus infection and chronic kidney disease: time for reappraisal. J. Hepatol. 65, S82–S94 (2016).
Sise, M. E. et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 63, 408–417 (2016).
Merli, M., Carli, G., Arcaini, L. & Visco, C. Antiviral therapy of hepatitis C as curative treatment of indolent B cell lymphoma. World J. Gastroenterol. 22, 8447–8458 (2016).
Peveling-Oberhag, J., Arcaini, L., Bankov, K., Zeuzem, S. & Herrmann, E. The anti-lymphoma activity of antiviral therapy in HCV-associated B cell non-Hodgkin lymphomas: a meta-analysis. J. Viral Hepat. 23, 536–544 (2016).
Arcaini, L., Rossi, D. & Paulli, M. Splenic marginal zone lymphoma: from genetics to management. Blood 127, 2072–2081 (2016).
Tilly, H. et al. Diffuse large B cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26, v116–v125 (2015).
Musto, P., Dell’Olio, M., Carotenuto, M., Mangia, A. & Andriulli, A. Hepatitis C virus infection: a new bridge between hematologists and gastroenterologists? Blood 88, 752–754 (1996).
Carrier, P. et al. HCV-associated B cell non-Hodgkin lymphomas and new direct antiviral agents. Liver Int. 35, 2222–2227 (2015).
Merli, M. et al. Outcome prediction of diffuse large B cell lymphomas associated with hepatitis C virus infection: a study on behalf of the Fondazione Italiana Linfomi. Haematologica 99, 489–496 (2014).
Kyvernitakis, A. et al. Hepatitis C virus infection in patients undergoing hematopoietic cell transplantation in the era of direct-acting antiviral agents. Biol. Blood Marrow Transplant. 22, 717–722 (2016).
Arcaini, L. et al. Interferon-free antiviral treatment in B cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood 128, 2527–2532 (2016).
Dlouhy, I. et al. Clinico-biological characteristics and outcome of hepatitis C virus-positive patients with diffuse large B cell lymphoma treated with immunochemotherapy. Ann. Hematol. 96, 405–410 (2017).
Vallisa, D. et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J. Clin. Oncol. 23, 468–473 (2005).
Braun, G. S., Horster, S., Wagner, K. S., Ihrler, S. & Schmid, H. Cryoglobulinaemic vasculitis: classification and clinical and therapeutic aspects. Postgrad. Med. J. 83, 87–94 (2007).
Reviewer information
Nature Reviews Disease Primers thanks F. Dammacco, Y. Shoenfeld, L. Quartuccio and the other anonymous referee(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (D.R.); Epidemiology (A.G.T.); Mechanisms/pathophysiology (D.R. and P.C.); Diagnosis, screening and prevention (C.F. and D.S.); Management (F.C.F., M.R.-C. and A.L.Z.); Quality of life (M.R.-C.); Outlook (D.R.); Overview of Primer (D.R.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roccatello, D., Saadoun, D., Ramos-Casals, M. et al. Cryoglobulinaemia. Nat Rev Dis Primers 4, 11 (2018). https://doi.org/10.1038/s41572-018-0009-4
Published:
DOI: https://doi.org/10.1038/s41572-018-0009-4
This article is cited by
-
Ocular manifestations of cryoglobulinemia: a reappraisal
Eye (2024)
-
Geschlechtsspezifischer Verlauf der Nierenbeteiligung bei Vaskulitiden
Die Nephrologie (2024)
-
Vasculitic Neuropathies
Current Treatment Options in Neurology (2024)
-
Sekundäre Vaskulitiden
Die Innere Medizin (2024)
-
Coexistence of cryoglobulinemia and ANCA-associated vasculitis in a chronic brucellosis patient -a case report and literature review
BMC Infectious Diseases (2023)